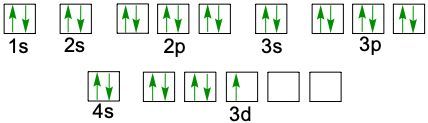
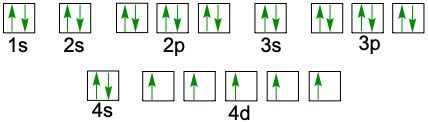
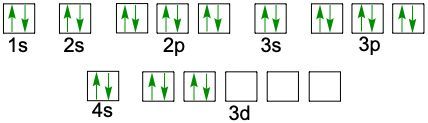
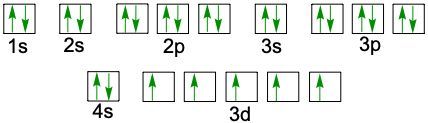
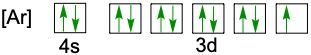
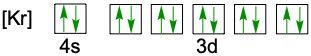
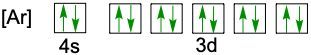
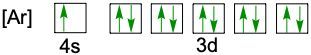
When writing the electron configuration of an element, it's important that it represents the distribution of electrons from S1 to S2 to P2 and so on within orbitals using the off bow principle. Now with the off bow principle it says starting with S1 we're going to have electrons filling lower energy orbitals before moving on to higher energy orbitals. We begin with S1 when we're doing the full ground state electron configuration of an element or an ion.
To help us with this, you could take the off bound diagram approach. We start out with S1, then we move on, loop back around to S2 then we loop back around to P2, then to S3 Then we loop back around to P3 and then S4. Then continuously loop back around to D3 to P4 to S5. Now this is our off bound diagram.
In the off bound diagram we have here S1 all the way down to S8 Then we have P2 to three on to seven P7 then we have D3 to D6 and then we have F4 and F5. Another way we can look at determining the electron configuration has to do more with the periodic table. So if you click on the next video, let's reimagine what the periodic table will look like when dealing with the electron configuration of elements and ions.