Here we're told when 60 grams of lead at 68.3�C is poured into 90 grams water at 30�C within a coffee cup calorimeter, the temperature increases to 48�C. Based on this information, what is the heat capacity of the calorimeter? We're told the specific heat capacity, specific heat of lead and water are .128 and 4.184 respectively.
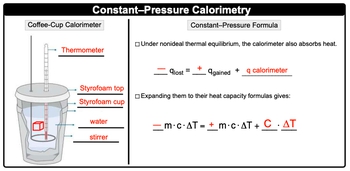
All right, so we're going to plug. Well, first of all, we need to realize we have our container with water and we're placing it lacing into it. Our lead lead is at a higher temperature than the water is. Remember, the hotter object always releases its heat, so it's going to release its heat. So the water is releasing its heat. So negative Q of water. Well, no negative Q of lead. Sorry. The object that's releasing its heat equals positive Q of water, which is absorbing it, plus Q of the calorimeter.
The calorimeter is absorbing some of it as well because they tell us that we need to find AT capacity. We don't need to find AT capacity if it was involved within the calculation, All right. So if their queues are involved, that means their mcats for some of them are involved. So negative MCAT of our lead equals positive MCAT of the water. Remember calorimeters? Their masses tend to be unknown, so it's just the heat capacity that they have times the change in temperature.
All right, So here we're going to say -60 grams of our lead times its specific heat capacity times the change in temperature. We're told that the temperature increases to 48�C, that the final temperature for everyone O. Here the change in temperature for the lead is the final temperature minus its initial temperature of 68.3�C that equals positive. And cat of water and cat on. So water is 90 grams of water times its specific heat capacity times its final temperature minus the initial temperature that it had.
Plus, we don't know the heat capacity of the calimeter, and that's what we're looking for. And here's the thing, before we add the lead to the water, it was just the water in the calorimeter hanging out with each other, so they both must have been at the same initial temperature. So change in temperature would be 48�C minus the initial temperature of the calorimeter, which again it was with the water, and we assumed they both were at the same initial temperature of 30�C.
All we have to do now is solve for this one missing variable. So what we're going to do now is we're going to multiply everything here on this side. Grams will cancel out, degrees Celsius will cancel out. We'll have Joules left on this side, which comes out to 155.904 joules equals grams cancel out, degrees Celsius cancel out. So we're going to have everything here also in joules. So that is 6778.08 Joules. Plus, when you subtract these, that's going to give me 18�C times my specific capacity or my heat capacity of C.
We got to isolate our heat capacity of C So next we're going to subtract this from both sides here. So when we do that, we're going to have now negative 66.2217600. Actually negative 6622.176 equals 18�C times my heat capacity of C divide out 18.0�C. So remember jewels are still here, so one 8.0�C heat capacity is a number that is positive. So this is an absolute terms. When we do that, that's Joules divided by degrees Celsius. So here my heat capacity which is capital C = 367.9 Joules over degrees Celsius. So that would be the heat capacity of our calorimeter within this given question.