Solutions represent homogeneous or homogeneous mixtures made-up two or more components that form a uniform composition. Now when we say uniform composition, that means that everything mixes perfectly together. So when I take a look at the result, I can't tell which part is which. Everything is mixed perfectly together.
Now when it comes to solutions, we have portions of it that we need to understand. First we have our solute. The solute represents the smaller portion of the solution that is dissolved within the solvent. The solvent itself is the part of the solution that is present in the larger amount, and it can dissolve other substances.
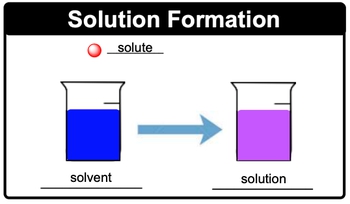
Concentration is a word that you will hear. Oftentimes, concentration is basically a measurement of the amount of solute that you have present in a given solution. So speaking about solutions, let's take a look at this solution formation image. The smaller ball there, it's smaller in amount, so it represents our solute. This solute, I'm going to dunk it into this solvent. Let's just assume it's water which is larger in amount.
Then you need to realize when you have a solute dissolving in a solvent they create a purple solution in this case which is our solution. So just remember, solutions are homogeneous mixtures and it's solute added to solvent that helps to create them.