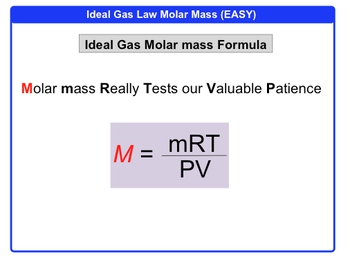
Here we're told calculate the molar mass of a gas if 2.50g occupies 0.995 liters at 715 Tor and 40�C. All right, we need to calculate molar mass. OK, now, molar mass here equals grams over moles. So let's just say that grams over moles within the question, they give us grams. Right off the bat, we have 2.50 grams, but we don't have the moles.
However, in the question we see that we have volume, we have pressure, and we have temperature. V&T, we know are part of the ideal gas law, so we're going to say PV=nRT. With this information we can isolate the moles of our gas. So divide both sides here by RT and we're going to say here that moles equals pressure times volume over RT. We need to convert our pressure into atmospheres.
So remember we have 715 tours and then we're going to stay here. For every one atmosphere that we have that's equal to 760 tours, tours here would cancel out and we get as our pressure 0.9408 atmospheres. Take those atmospheres and plug them in. So we have 0.9408 atmospheres. Our volume is already in liters, so we're good there. Remember R is our gas constant which is 0.08206 liters times atmospheres over moles times K and temperature.
Temperature has to be in Kelvin O. Add 273.15 to this. So when we do that, that's going to give us 313.15 Kelvin. Plug that in. What units? Cancel out, Atmospheres cancel out, liters cancel out, Kelvin's cancel out. And that's how we see we'll have moles at the end. So here when we do that, we get our moles as 0.0364 moles, take those moles and plug them into the molar mass formula, and doing that gives us a value of 68.681g per mole.
Now here 2.50 has three sig figs, 3 sig figs, 3 sig figs, 40 has only one sig fig. So if we wanted to go by that one sig fig, that will come out to 70 grams per mole. It's not as accurate, but it's not a big deviation from our initial answer. So if we're going by significant figures, we can say that 70 grams per mole would be the molar mass of our unknown gas.