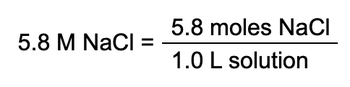In this example question, it says how many grams of sodium phosphate, which has a molecular mass of 163.94 grams per mole, are present in 300 milliliters of a .550 molar sodium phosphate solution. All right, so in this question they're asking us to discover grams. Now, before we start plugging in numbers and doing calculations, let's talk about what the sentence is saying and how we need to be on the look at when we see it written in such a way.
Now here we have a number and here we have another number and they are connected together by the word of. So just remember when of is in between two values of is really telling us to multiply them together. Now we have molarity here which remember molarity equals moles over liters and realize this that if I were to multiply both sides by liters then we would see that moles equals liters times molarity. Look at this, this is milliliters. If I were to convert those into liters and then multiply it by the molarity, that would give me moles of sodium phosphate.
And we know if we have moles of a compound, we can use its molecular mass or molar weight to help us determine the grams of that compound. So that's the key. So just remember here when we have volume of molarity, that's really just telling us, hey you have moles there, all right. So we're going to first take the 300 milliliters and I'm going to convert it into liters. Remember this is a metric prefix conversion, so milliliters go on the bottom, liters go on top. Remember that the metric prefix is on the same side with one, so 1,000,000 is 10 to the -3 milliliters cancel out. Now I have liters.
Also remember that molarity is a conversion factor, so that .550 molar is really saying that I have 0.550 moles of that compound over one liter, so I need liters to cancel out. So I'll put the one liter here and then we have 0.550 moles of sodium phosphate here. Liters cancel out and look, I have moles of sodium phosphate. Finally, I convert those moles into grams. So one mole of sodium phosphate. We're told that the molecular weight is 163.94 grams. So plug that in so moles cancel out and I'll be left with grams at the end.
So initially what I'll get is 27.0501 grams of sodium phosphate. Looking at the question, 300.0 has four sig figs, .550 has three sig figs, and this number here has five sig figs. We go with the least number of significant figures, so this comes out to be 27.1g of sodium phosphate. So this would be the grams of our compound. So just remember, when they're giving us volume of molarity, they're telling us what the moles are here. In this case, we just had to find grams. So take those moles that you've isolated and convert them into grams and you'll have your final answer.