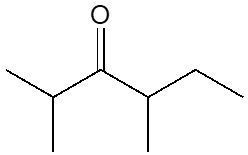
Here it says provide the formal name for the ketone shown. Now to do that we're going to use the following steps. TE one is find the longest carbon chain which represents our parent chain and assign name according to the prefixes and the modifier. Now the parent change should include the carbonyl group. So remember your carbonyl carbon or carbonyl group is this C2=O and have greater number of carbons. If a tie between longest chain choose chain with more substituents.
If we take a look here, our longest carbon chain will be this portion here. Now assign name to all substituents for Step 2. Our substituent here would be this carbon that's branching off of the main chain. This would be a methyl group. Step three, start numbering the chain from the end closest to the carbonyl carbon. So we have to start numbering from this end 123-4567 and eight.
If a time the number from the end closest to the next substituent, it's still a tie number in alphabetical order in terms of the substituents. And then here assign location to the carbonyl carbon, the carbonyl carbons on carbon #2. All right. And then we repeat steps 4:00 to 6:00. And these are from previous naming topics mainly. If we're looking at alkanes with substituents, that's where we're coming up with these rules.
Or steps 4:00 to 6:00, if you haven't watched that video, make sure you go back and take a look because in it we talk about our substituents. They're written at the beginning of the name. We have to give their location numerical location and if there's multiple substituents, we have to name them alphabetically. So here, if we take a look, we're going to say our substituent is on carbon 5, so it's 5 methyl. The carbonyl carbon is on carbon. 2SO28 carbons is octane, but we change the E ending to 1 so it becomes 2 octanone. So this would be the name of this particular ketone 5 methyl 2 octanone.