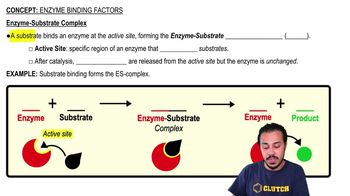
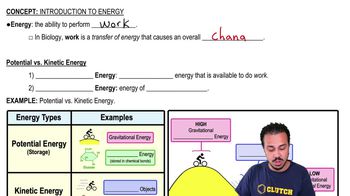
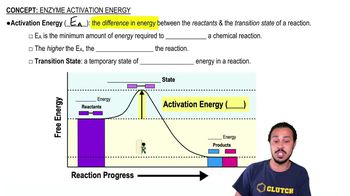
Use the following choices to answer questions 7–10.
a. E. coli growing in glucose broth at 35℃ with O₂ for 5 days
b. E. coli growing in glucose broth at 35℃ without O₂ for 5 days
c. both a and b
d. neither a nor b
Which culture uses the most glucose?