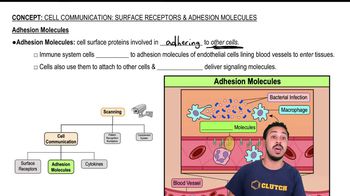
What type of bond holds the following atoms together?
a. Li+ and Cl- in LiCl
b. carbon and oxygen atoms in methanol
c. oxygen atoms in O₂
d. a hydrogen atom of one nucleotide to a nitrogen or oxygen atom of another nucleotide in:
<IMAGE>
 Verified step by step guidance
Verified step by step guidance

What type of bond holds the following atoms together?
a. Li+ and Cl- in LiCl
b. carbon and oxygen atoms in methanol
c. oxygen atoms in O₂
d. a hydrogen atom of one nucleotide to a nitrogen or oxygen atom of another nucleotide in:
<IMAGE>
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. This process is the basis for questions 1-3.
If E. coli were grown in a medium containing the radioactive isotope ³²P, the ³²P would be found in all of the following molecules of the cell except
a. ATP.
b. carbohydrates.
c. DNA.
d. plasma membrane.
e. complex lipids.
Classify the following as subunits of either a carbohydrate, lipid, protein, or nucleic acid.
a. <IMAGE>
b. <IMAGE>
c. <IMAGE>
d. Thymine nucleotide
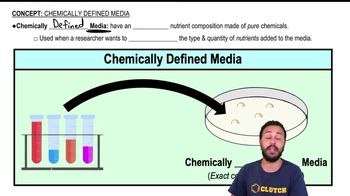
Classify each of the molecules on the left as an acid, base, or salt. The dissociation products of the molecules are shown to help you.
HNO₃ →H⁺ + NO⁻₃
Classify each of the molecules on the left as an acid, base, or salt. The dissociation products of the molecules are shown to help you.
H₂SO₄ →2H⁺ + SO²₄⁻
DRAW IT The following diagram shows the bacteriorhodopsin protein. Indicate the regions of primary, secondary, and tertiary structure. Does this protein have quaternary structure?
<IMAGE>