Here are the essential concepts you must grasp in order to answer the question correctly.
Acids and Bases
Acids are substances that can donate protons (H⁺ ions) in a solution, while bases are substances that can accept protons. The Brønsted-Lowry theory defines acids as proton donors and bases as proton acceptors, which is essential for understanding the behavior of molecules in aqueous solutions.
Recommended video:
Dissociation
Dissociation refers to the process by which a compound breaks down into its constituent ions in solution. For example, when nitric acid (HNO₃) dissociates, it produces H⁺ and NO₃⁻ ions, which is crucial for classifying the compound as an acid based on its ability to release protons.
Recommended video:
Transcription Termination in Prokaryotes
Salts
Salts are ionic compounds formed from the neutralization reaction between an acid and a base. They typically consist of cations from the base and anions from the acid. Understanding the formation of salts helps in classifying compounds based on their dissociation products and their role in acid-base chemistry.
Recommended video:
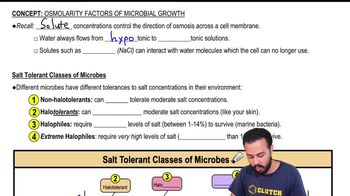
Osmolarity Factors for Microbial Growth