Textbook Question
How are the following pairs of carbohydrates, shown in a Fischer projection, related to each other? Are they structural isomers, enantiomers, diastereomers, or epimers? Identify each as the d- or l-isomer.
(b) <IMAGE>
15
views
 Verified step by step guidance
Verified step by step guidance


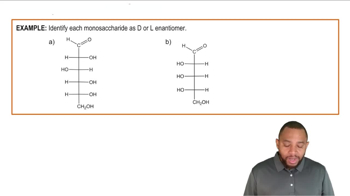
How are the following pairs of carbohydrates, shown in a Fischer projection, related to each other? Are they structural isomers, enantiomers, diastereomers, or epimers? Identify each as the d- or l-isomer.
(b) <IMAGE>
Identify the following carbohydrates as the α or ß anomer:
(a) <IMAGE>
Identify the following carbohydrates as the α or ß anomer:
(b) <IMAGE>
Draw the product of the following 1→4 condensation and name the glycosidic bond:
<IMAGE> + <IMAGE> →
Isomaltose, a disaccharide formed during caramelization in cooking, contains two glucose units bonded α (1→6) . Draw the structure of isomaltose.
d-Fructose can also form a six-membered ring. Draw the anomer of d-fructose in the six-membered ring form.