Draw the Fischer projection of the product of the oxidation of d-galactose at C1.
Ch.6 Carbohydrates–Life’s Sweet Molecules
Chapter 3, Problem 6.92
d-Fructose can also form a six-membered ring. Draw the anomer of d-fructose in the six-membered ring form.
 Verified step by step guidance
Verified step by step guidance1
Identify the structure of d-fructose in its linear form. d-Fructose is a ketohexose, meaning it has six carbon atoms and a ketone group at the second carbon.
Recognize that the six-membered ring form of d-fructose is called a pyranose form. This occurs when the hydroxyl group on the fifth carbon (C5) attacks the carbonyl carbon (C2), forming a hemiacetal.
Determine the stereochemistry at the anomeric carbon (C2) after the ring closure. The anomeric carbon is the new chiral center formed during the cyclization. The two possible configurations are alpha (α) and beta (β).
Draw the six-membered ring structure, ensuring that the oxygen atom is part of the ring. Place the substituents (hydroxyl groups and hydrogen atoms) on the ring according to the stereochemistry determined in the previous step.
Label the anomeric carbon and indicate whether the structure is the alpha or beta anomer. In the alpha anomer, the hydroxyl group on the anomeric carbon is trans to the CH2OH group, while in the beta anomer, it is cis.

Verified Solution
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Anomer
An anomer is a type of stereoisomer that differs at the anomeric carbon, which is the carbon atom that becomes a new chiral center when a sugar cyclizes. In the case of d-fructose, the anomeric carbon is the one that was originally the carbonyl carbon in the open-chain form. The two anomers are designated as alpha (α) and beta (β) based on the orientation of the hydroxyl group attached to the anomeric carbon.
Recommended video:
Guided course

Cyclic Structures of Monosaccharides Concept 1
Cyclization of Sugars
Cyclization is the process by which a linear sugar molecule forms a cyclic structure, typically a five- or six-membered ring. For d-fructose, this involves the reaction of the carbonyl group with a hydroxyl group on the same molecule, leading to the formation of a hemiacetal. This process is crucial for understanding the structural diversity of sugars and their reactivity.
Recommended video:
Guided course

Ketoses as Reducing Sugars Example 2
Haworth Projection
The Haworth projection is a common way to represent cyclic sugars in a two-dimensional format that illustrates the cyclic structure and the orientation of substituents. In this representation, the ring is depicted as a planar structure, with substituents drawn above or below the plane to indicate their orientation. This format is particularly useful for visualizing the anomeric carbon and distinguishing between the alpha and beta forms of sugars.
Recommended video:
Guided course
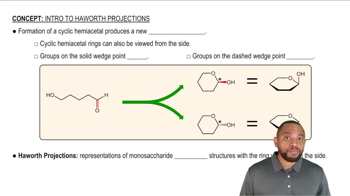
Intro to Haworth Projections Concept 1
Related Practice
Textbook Question
28
views
Textbook Question
Draw the product of the following 1→4 condensation and name the glycosidic bond:
<IMAGE> + <IMAGE> →
8
views
Textbook Question
Isomaltose, a disaccharide formed during caramelization in cooking, contains two glucose units bonded α (1→6) . Draw the structure of isomaltose.
10
views
Textbook Question
Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a) <IMAGE>
17
views
Textbook Question
Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a) <IMAGE>
9
views
Textbook Question
Draw the Fischer projection for the enantiomer (mirror image) of each of the following:
(a) <IMAGE>
d-Altrose
10
views
