Textbook Question
The most prevalent fatty acid in coconut oil is lauric acid, a saturated fatty acid containing 12 carbons. Draw lauric acid in skeletal structure.
9
views
 Verified step by step guidance
Verified step by step guidance
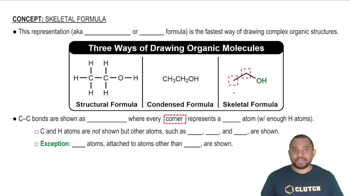

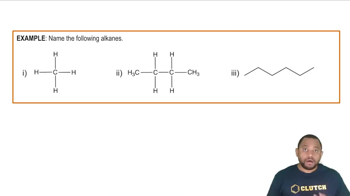
The most prevalent fatty acid in coconut oil is lauric acid, a saturated fatty acid containing 12 carbons. Draw lauric acid in skeletal structure.
Draw the condensed structural formula for each of the following alkyl groups: (b) methyl
Draw the skeletal structure for each of the following compounds:
(a) 2,3-dimethylpentane
Give the correct IUPAC name for each of the following compounds:
(a) <IMAGE>
Give the correct IUPAC name for each of the following compounds:
(c) <IMAGE>
What is the difference between a conformational isomer of a compound and a structural isomer of the same compound?