Here are the essential concepts you must grasp in order to answer the question correctly.
Alkyl Groups
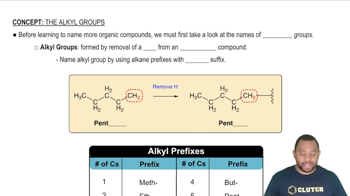
Alkyl groups are derived from alkanes by removing one hydrogen atom, resulting in a functional group that can bond with other atoms. They are characterized by the general formula CnH2n+1, where 'n' represents the number of carbon atoms. Methyl, for example, is the simplest alkyl group with one carbon atom (C1) and three hydrogen atoms (H3), represented as -CH3.
Recommended video:
Condensed Structural Formula
A condensed structural formula is a way of representing a chemical structure that shows the arrangement of atoms in a molecule without depicting all the bonds explicitly. It simplifies the representation by grouping atoms together, making it easier to visualize the structure. For methyl, the condensed structural formula is simply written as CH3, indicating one carbon atom bonded to three hydrogen atoms.
Recommended video:
Condensed Formula Concept 1
Structural Representation
Structural representation in chemistry refers to the various ways of depicting the arrangement of atoms within a molecule. This can include Lewis structures, condensed formulas, and skeletal formulas. Understanding these representations is crucial for interpreting chemical structures and reactions, as they provide insight into the connectivity and functional groups present in the molecule.
Recommended video:
Molecular Representations Concept 1
 Verified step by step guidance
Verified step by step guidance