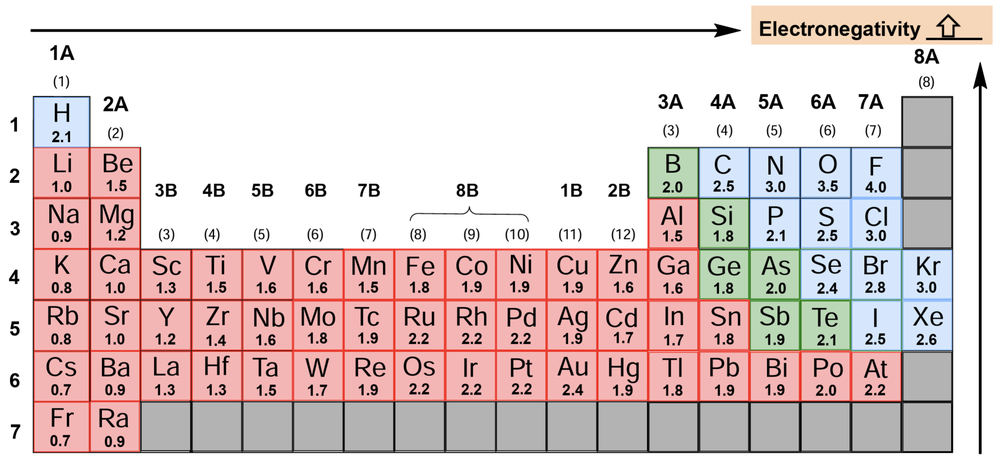
Electronegativity is a crucial concept in chemistry, defined as the ability of an element to attract electrons towards itself. The higher the electronegativity value of an element, the greater its tendency to draw electrons in. This concept was notably advanced by American chemist Linus Pauling in 1932, who established a scale for electronegativity values across the periodic table.
Understanding the periodic trends of electronegativity is essential. As one moves from left to right across a period and from bottom to top within a group on the periodic table, electronegativity values generally increase. This trend indicates that elements located towards the top right corner of the periodic table, particularly nonmetals, exhibit higher electronegativity. Notably, fluorine is recognized as the most electronegative element, assigned a value of 4.0 on Pauling's scale, which can be remembered as the ideal GPA that many students aspire to achieve.
While noble gases typically do not seek additional electrons, exceptions exist with elements like krypton and xenon, which can accommodate extra electrons under certain conditions. This nuanced understanding of electronegativity is vital for predicting how atoms will interact in chemical bonding, particularly in the formation of polar covalent bonds where differences in electronegativity between bonded atoms lead to dipole moments.