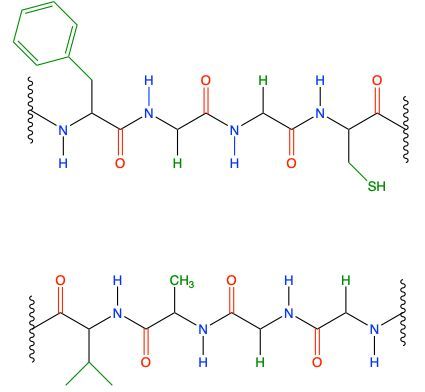
Now, when it comes to the secondary protein structure, we're going to say that this is the type of structure that results from hydrogen bonding of the atoms in the backbone of a protein. We're going to say it involves the connection between the amide hydrogen of one peptide with the carbonyl oxygen of another. So if we take a look here at this image, we're going to say that this is our peptide chain 1, and this is our peptide chain 2. Here we're going to say, we have this is an amide here, this NH group, because it's connected to a carbonyl, we just don't see it. It's what's connected to it out of view. And this is our carbonyl oxygen. They will form a hydrogen bond with one another. Remember, hydrogen bonding is not actually bonding; it's an intermolecular force. So we're going to show it and depict it through dashed bonds, so dashed lines. So that's one hydrogen bond. Here goes another amide nitrogen. Again, it's an amide because it's connected to this carbonyl here. So there goes the amide hydrogen, the carbon, and the carbonyl oxygen. So there goes another hydrogen bond. And here we just have two R groups. We don't specify what kinds of R groups they are, so we don't know if hydrogen bonding would happen, so we don't do anything. So in this example, we have two sites where we see hydrogen bonding being used. Alright, so just remember, it's this type of hydrogen bonding that exists, that happens, that helps to give us our secondary protein structure.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Secondary Protein Structure: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AISecondary protein structures arise from hydrogen bonding between amide hydrogens and carbonyl oxygens in peptide chains, forming two main patterns: the alpha helix and beta pleated sheet. The alpha helix adopts a right-handed spiral shape, with 3.6 residues per turn, while the beta pleated sheet consists of zigzagging beta strands oriented side by side. In both structures, R groups extend outward, optimizing spatial arrangement. Understanding these formations is crucial for grasping protein functionality and stability.
Secondary Protein Structure Concept 1
Video transcript
Secondary Protein Structure Example 1
Video transcript
Determine which of the following amino acid pairs could potentially perform hydrogen bonding between their respective r groups.
For option a, we have Glycine and Serine together. Glycine has an H as its R group. Because of the presence of an H, there's no way that it could form hydrogen bonding with its r group with serine. So this is out as a possibility.
Next, we have Aspartic acid and Glutamic acid. These are acidic amino acids. They possess carboxylic acids within their R groups. Because of this, they have the potential to hydrogen bond. So, we say that this could perform hydrogen bonding between their respective R groups.
Next, we have Valine and Leucine. These two represent non-polar amino acids. Their r groups are hydrocarbons. They're only composed of carbons and hydrogens, so they wouldn't be able to engage in hydrogen bonding with their r groups. So this is out.
Then finally, we have Aspartic acid again, and then we have Arginine as our other amino acid. Aspartic acid is an acidic amino acid. Arginine is a basic amino acid. It can exist as a protein as an amino group that has an H already on it, or it can exist in its neutral form. Either way, because one is acidic and one is basic, they could engage in hydrogen bonding between their respective R groups. So this is also a possibility.
In this case, we can say both options B and D could engage in hydrogen bonding between their respective r groups. Right. So the answer again here would be options B and D.
How many hydrogen bonding pairs are possible when the following two peptides interact?
3
5
4
6
1
Alpha-Helix Concept 2
Video transcript
Secondary structures give rise to 2 types of repeating patterns. In this video, we're going to take a look at the alpha helix. Now, you're going to say that the backbone of a single protein chain coils into a spiral-like staircase. If we take a look at the image to the right, we have our primary structure, which remember is just a sequence of amino acids depicted by these beads here that are connected to each other through peptide bonds. This chain interacts with itself and it coils to give us our first secondary structure, this alpha helix. Here, we can see that it becomes spiral-like. Now, there's a second structure, we're going to talk about that later on, but for right now, we're talking about the alpha helix. Now, when it comes to the alpha helix, we're going to say it's stabilized by hydrogen bonding between distant amino acids on the same chain. So again, this is just that one chain hydrogen bonding with itself to make this staircase. If we took a look here, here goes our chain that's coiled up. And when it comes to hydrogen bonding, remember, it happens between the amide hydrogen and the carbonyl oxygen. So we'd have our hydrogen bond here connecting these two. We'd have another hydrogen bond here connecting these two. So here we're showing two places of hydrogen bond formation. This alpha helix can just be one region of a large polypeptide chain. Here, if we take a look to the right, we've highlighted these two alpha helices here, this one and this one. And as you can see, they're just a small segment of the entire chain. The rest of the chain is larger than it and it's grayed out. So again, a secondary structure is just the chain interacting with itself. In this case, it's hydrogen bonding with itself to help make this staircase. This represents one of our first of 2 repeating patterns that help to make a secondary structure for a protein.
Secondary Protein Structure Example 2
Video transcript
Determine which of the following statements represents a secondary structure for a protein. Here, creation of peptide bonds. Remember, creation of peptide bonds helps to make the sequence of amino acids and the primary structure. Attractive force between the H atom of a peptide bond and the oxygen atom of a peptide bond. Alright. So here, this makes the most sense because it's basically saying we have hydrogen bonding that is happening. This is one of the forces that helps to make the staircase and create our alpha helix. So, option B is correct. In my bond formation, the creation of an amino acid chain, this is saying the same thing as option A. We're talking about a primary structure when we're talking about peptide bonds or amide bond formation.
Ionic bond formation between the R side chains of alanine and valine. So here, this has nothing to do with secondary structure. We never talked about ionic bond formation, so this would not work. Also, we don't need to go into the discussion of what kinds of R groups these two amino acids have. Can they even undergo ionic bond interactions? That doesn't matter. We know that ionic bond formation was not one of the criteria to help make this staircase when it comes to the alpha helix and secondary structures of a protein. So here, only option B is the correct answer.
Alpha Helix Spiral Shape Concept 3
Video transcript
Now when it comes to the alpha helix, we can say that it adopts an optimal shape. Here, we're going to say that the spiral-like staircase adopts a right-handed or clockwise shape. This is basically just how the staircase twists and turns. Which direction does it take? We'd say that this is a right-handed or clockwise direction, i.e., shape. Now here we're going to say that the hydrogen bonds lie within the helix. So remember, we have hydrogen bondings that are helping to stabilize this structure. So we show these dotted lines that are helping to create the staircase, and the amino acid R groups lie outside the helix because of spacing. So if we take a look at these two images, we'd say here that this represents our right-handed alpha helix. We have our hydrogen bonding that's happening in between. And then here, we have our R groups. Because of spacing, they can't be inside of the staircase or the helix; they'd be represented outside of it. So we'd have an R group here, here, and in all of these places.
Now, in addition to this, we can say that the hydrogen bonding of the amide hydrogen and the carbonyl oxygen happens or residues further on the helix. Now, what is the result of this? Well, the result is that for every one turn of the helix, it contains an average of 3.6 residues. This is going to become important because they could ask you questions in terms of how many turns you would have if you have this many number of residues or if you have this many number of residues, how many theoretical turns could your alpha helix possess? Right. So there's a connection: for every one turn, there are 3.6 residues on average. Right. So keep this in mind when we talk about alpha helices, their optimal shapes, and the number of residues per turn.
Secondary Protein Structure Example 3
Video transcript
Here it says, what is the maximum number of turns for an alpha helix that contains 72 residues? Alright. So we have 72 residues within this question. Now remember, we have a conversion factor. Our conversion factor says that for every one turn, we have 3.6 residues involved. We're going to use this to help us find our answer. We want to cancel out residues, so we put that on the bottom. So we have 3.6 residues here on the bottom, and that's for every one turn that's on top. Here, my residues would cancel out, and at the end, I'd have my number of turns. So my end amount will come out to be 20 turns. So here, this would mean that our answer would have to be option d, 20 turns.
Beta-Pleated Sheet Concept 4
Video transcript
So now remember that secondary structures give rise to two repeating patterns. Here, we're going to talk about the second pattern, which is called our beta pleated sheet. Now, here, this is a secondary structure consisting of two or more beta strands oriented side by side. If we take a look to the right, we see that, again, our primary structure comes from the connecting of amino acids in a sequence through peptide bonds. We know that it can give rise to an alpha helix as a secondary structure, but now we're going to talk about a beta pleated sheet as another secondary structure. Now, the name pleated is because of their zigzag pattern. If we take a look here at the sheet. If we really visualize this, we have our two peptide chains that are hydrogen bonding to one another. Remember, here we have hydrogen bonding happening here, here, here, and here. So I've basically identified hydrogen and also here, and that locks in here. So, these are all our spots of hydrogen bonding. Got all of them? Yes. So, these are all our sites of hydrogen bonding that connect these two peptide chains to one another, and we can see that these two chains that are hydrogen bonding to each other, they kind of reside, if you look at it, like on a sheet on a full piece of paper. That's what we talk about pleated, the zigzagging pattern. Think of paper being folded. The way the two chains orient themselves because of this hydrogen bonding, because of this way the beta strands orient themselves side by side gives us this depiction of a sheet. Now, here, we're talking about the chains, but remember, there are R groups. R groups, because of spacing, can't be in the interior. That's where hydrogen bonding is occurring. So what they do is they orient themselves above and below this sheet. So we're going to say the R groups, they're going to extend above or below the beta sheet. So here we have the R groups on top, so they're above the sheet, and then we have these R groups on the bottom below the sheet. And this all has to do with spacing and taking the optimal shape when it comes to these peptide chains. Alright. So remember, we have our primary structure. The primary structure leads to our secondary structure. This is happening to create two different types of possible shapes where we have alpha helices and we have beta sheets.
Secondary Protein Structure Example 4
Video transcript
Which of the following statements is true of beta sheets? Interchanging between an alpha helix and a beta sheet is a key feature of a primary structure. Alright. So here, we never talked about interchanging between alpha and beta. We talked about these being two possible repeating patterns. Also, we talked about them being key features of secondary structure. Okay. So, this would be wrong for those two reasons. Their interior is characterized by hydrogen bonding between amide hydrogens and carbonyl oxygens. We've seen this. That's what allows these two portions of these chains to interact with each other. So, their interior is characterized by r side chains interactions. No. The r groups orient themselves above and below the sheet. That's what happens when we do these beta sheets in terms of our secondary structure of proteins. The r side chains extend inward to ensure greater packing of the peptides. It's the opposite. The R groups take up space and optimally we want them to be oriented on the outside. So here, the only answer that is correct would have to be option b.
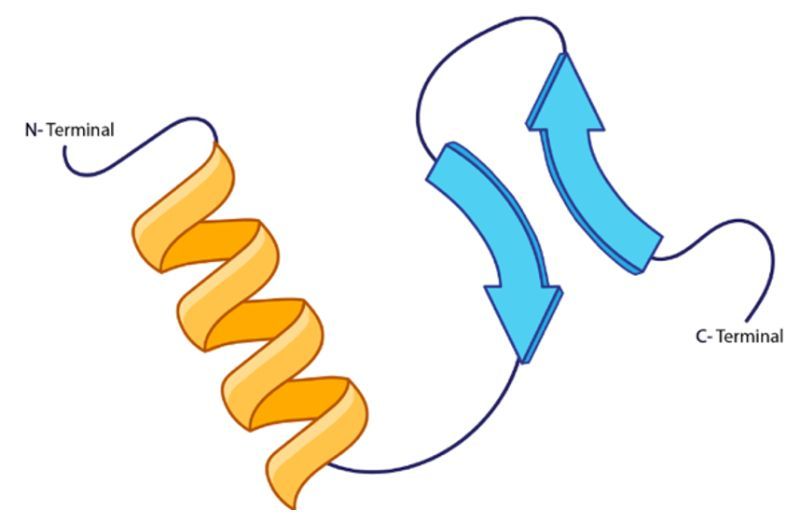
Which of the following statements is true in regard to the peptide strand shown?
The β-sheet defines the primary structure of the peptide strand.
The C-Terminal end possesses an α-helix.
Along with its α-helix counterpart, the β-sheet is mainly stabilized by backbone hydrogen bonds.
The α-helix and the β-sheet are connected together through an ionic bond.
Do you want more practice?
Here’s what students ask on this topic:
What is the secondary structure of a protein and how is it formed?
The secondary structure of a protein refers to the local folded structures that form within a polypeptide due to interactions between atoms in the backbone. It is primarily formed through hydrogen bonding between the amide hydrogen (NH) and the carbonyl oxygen (C=O) of the peptide bonds. The two main types of secondary structures are the alpha helix and the beta pleated sheet. The alpha helix is a right-handed coil stabilized by hydrogen bonds between every fourth amino acid, while the beta pleated sheet consists of beta strands connected side by side through hydrogen bonds, forming a zigzag pattern. These structures are crucial for the protein's overall stability and function.
 Created using AI
Created using AIWhat are the characteristics of an alpha helix in protein secondary structure?
An alpha helix is a common secondary structure in proteins characterized by a right-handed, spiral-like shape. It is stabilized by hydrogen bonds between the amide hydrogen and the carbonyl oxygen of amino acids that are four residues apart. Each turn of the helix contains approximately 3.6 amino acid residues. The R groups of the amino acids extend outward from the helix, allowing for optimal spatial arrangement. This structure is crucial for the protein's stability and can be a segment of a larger polypeptide chain.
 Created using AI
Created using AIHow do beta pleated sheets form in protein secondary structure?
Beta pleated sheets form when two or more beta strands align side by side, connected by hydrogen bonds between the amide hydrogen and carbonyl oxygen of adjacent strands. This arrangement creates a zigzag pattern, resembling a pleated sheet of paper. The R groups of the amino acids in beta strands alternate, extending above and below the plane of the sheet. This structure provides stability and is essential for the protein's overall conformation and function.
 Created using AI
Created using AIWhat is the significance of hydrogen bonding in secondary protein structures?
Hydrogen bonding is crucial for the formation and stability of secondary protein structures. In alpha helices, hydrogen bonds form between the amide hydrogen and carbonyl oxygen of amino acids four residues apart, creating a stable helical structure. In beta pleated sheets, hydrogen bonds connect adjacent beta strands, forming a stable, zigzagging sheet. These hydrogen bonds are not true bonds but intermolecular forces that significantly contribute to the protein's overall stability and functionality by maintaining its specific shape.
 Created using AI
Created using AIHow do R groups influence the formation of secondary protein structures?
R groups, or side chains, influence the formation of secondary protein structures by affecting the spatial arrangement and stability of the alpha helix and beta pleated sheet. In alpha helices, R groups extend outward from the helical backbone, preventing steric clashes and allowing the helix to maintain its shape. In beta pleated sheets, R groups alternate above and below the plane of the sheet, optimizing spacing and reducing steric hindrance. The nature of the R groups (e.g., size, charge, hydrophobicity) can also influence the propensity of a polypeptide to form specific secondary structures.
 Created using AI
Created using AIYour GOB Chemistry tutor
- Examine the α-helix in Figure 18.1 and determine how many backbone C and N atoms are included in the loop betw...
- Consult the β-sheet in Figure 18.2 and (a) name the bonding responsible for the sheet formation and (b) identi...
- Draw the hexapeptide Asp-Gly-Phe-Leu-Glu-Ala in linear form showing all of the atoms, and show (using dotted l...
- Bradykinin, a peptide that helps to regulate blood pressure, has the primary structure Arg-Pro-Pro-Gly-Phe-Ser...
- What happens when a primary structure forms a secondary structure?
- What is the difference in hydrogen bonding between an α helix and a β−pleated sheet?
- What are some differences between each of the following pairs? (16.1, 16.2, 16.3)b. an α helix and collagen
- Name the stabilizing attractive force found in secondary structures of proteins.
- Describe the differences in the shape of an α helix and a β-pleated sheet.
- For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sh...
- For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sh...
- For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sh...