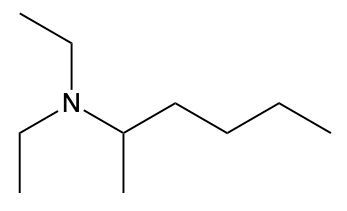
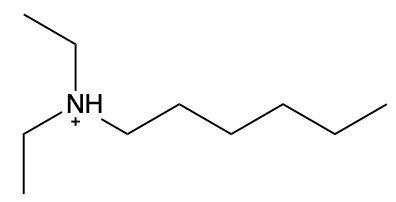
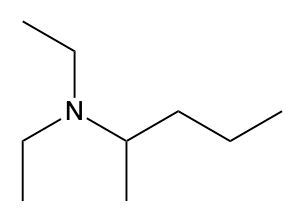
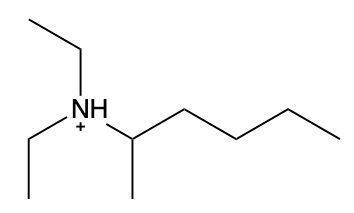
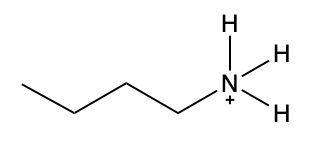
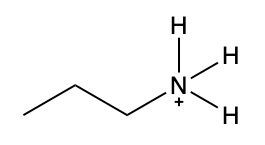
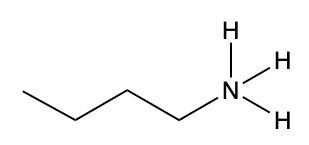
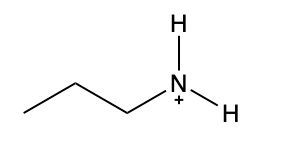
In this video, we're going to take a look at how to name ammonium salts. Now, ammonium salts, sometimes called ammonium ions, are compounds with one or more alkyl groups connected to a positively charged nitrogen atom. Now, here we're going to say the ammonium salts have a similar naming system to amines, whereas amines we say substituent and then we end the name with amine. Here, we're going to say substituent and end the name with ammonium ion. Now before we continue, I know that ammonium ion also exists as NH4+. We're not really concerned with that particular form of ammonium. What we're concerned with are the ones that are connected to carbons. All right. So that's what we're using the definition of an ammonium ion or salt as at least one alkyl group connected to a still positively charged nitrogen atom. So keep that in mind as we name these different types of structures.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Naming Ammonium Salts: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIAmmonium salts, characterized by one or more alkyl groups attached to a positively charged nitrogen atom, follow a naming convention similar to amines, with the suffix changing from "amine" to "aminium." To draw structures, ensure nitrogen makes four bonds, adding hydrogen atoms as needed. For naming, identify the largest carbon chain and list substituents alphabetically, using numerical prefixes for identical groups. This systematic approach is essential for understanding the properties and reactions of ammonium salts in organic chemistry.
Common Naming Concept 1
Video transcript
Drawing Ammonium Salts Concept 2
Video transcript
Now, in addition to knowing how to name ammonium salts, it's going to become important to know how to draw them. Recall that nitrogen has a plus one charge whenever it makes 4 bonds. Here in this example, it says, "Draw the structure for isopropylphenyl ammonium ion." Alright, so step 1 tells me, based on the name, to add the alkyl groups to the nitrogen atom. Here's the nitrogen. Connected to it is an isopropyl. Phenyl just means a benzene ring. Next, if the nitrogen atom isn't making 4 bonds, add enough hydrogen atoms until it does. So right now, nitrogen is only making 2 bonds. It needs to get to 4, so we add 2 hydrogens. And in step 3, we just add a plus one charge to the nitrogen atom because now it's making 4 bonds. So this would be the structure for our isopropylphenyl ammonium ion.
IUPAC Naming Concept 3
Video transcript
Now, when it comes to naming Ammonium salts, we're going to say the set of rules for naming Ammonium salts are similar to amines plus a modifier ending. Here, we're going to modify the ending from amine, which is what we'd have if we had an amine, to aminium, because it's an ammonium salt now. Just before we use the same naming convention, we designate the numerical locations of substituents. We talk about the location of the nitrogen, which forms the parent chain, and then we have the modified ending. We're going to think of it if it was an amine, it'd end with amine, but because it's a positive nitrogen, it's an ammonium ion. So amine changes to aminium.
So, if we take a look here, it says provide the systematic name for the following ammonium salt. Alright. So step 0 says that if it's symmetrical, we can follow the rules for naming them under common rules. But here it's not symmetrical, so we can ignore step 0. Step 1: if asymmetrical, we have to identify the largest carbon chain connected to the nitrogen atom and name it as an ammonium ion. So, if we take a look here, we have an isopropyl, a methyl, an ethyl, and a cyclopentane ring. The cyclopentane ring is the largest one. So here, we end it with that, so we'd say cyclo cyclopentane. But if it wasn't amine, it would end with amine. But here, we're really just changing the ending to aminium. So that's the end of the name.
Next, we're going to name other alkyl groups alphabetically. Oops. Okay. So, actually, use numerical location to indicate the location of nitrogen. Here, since it's on a ring, we don't have to state 1-cyclopentaminium. We don't have to do any of that stuff because it's on a ring. So we go straight to step 2. Name other alkyl groups alphabetically as N-substituents. If they are identical alkyl groups, use numerical prefixes (di, tri, etc.). Now, this grayed-out box is just something we've always done when it comes to naming, and it's used commas to separate numbers from numbers and uses dashes to separate letters from numbers. Letters are not separated from letters, something we've always done. So it's something you should always have in the back of your mind.
Now let's talk about these N-substituents. So what do we have here? We have methyl, we have ethyl, and we have isopropyl. So in alphabetical order, ethyl first. N-ethyl, then I next, N-isopropyl, N-methylcyclopentanaminium. It will be the name of the structure. I know it's a mouthful in terms of this, but this is the way we'd have to name this particular ammonium salt using the IUPAC naming system.
Draw structure for N,N-diethyl-2-hexanaminium.
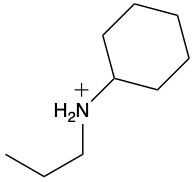
Draw structure for propylammonium ion.
Provide the IUPAC name for the following ammonium salt.
sec-butyl cyclohexyl ammonium ion
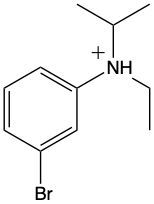
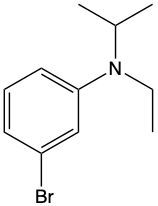
N-isopropylcyclohexanaminium
N-cyclohexyl-2-propanaminium
N-propylcyclohexanaminium
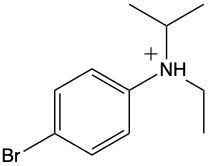
Which of the following represents m-bromo-N-ethyl-N-isopropylbenzenaminium?
Do you want more practice?
Here’s what students ask on this topic:
What are ammonium salts and how are they named?
Ammonium salts are compounds where one or more alkyl groups are attached to a positively charged nitrogen atom. The naming convention for ammonium salts is similar to that of amines, but with a key difference: the suffix changes from 'amine' to 'aminium.' For example, if you have an amine named 'methylamine,' the corresponding ammonium salt would be named 'methylaminium.' The systematic approach involves identifying the largest carbon chain connected to the nitrogen atom, naming it as the parent chain, and then listing other substituents alphabetically. Numerical prefixes are used for identical groups, and the nitrogen's location is indicated if necessary.
 Created using AI
Created using AIHow do you draw the structure of an ammonium salt?
To draw the structure of an ammonium salt, follow these steps: First, identify the alkyl groups attached to the nitrogen atom based on the name. For example, in 'isopropylphenylaminium ion,' you would attach an isopropyl group and a phenyl group (benzene ring) to the nitrogen. Next, ensure the nitrogen atom makes four bonds by adding hydrogen atoms if needed. Finally, add a +1 charge to the nitrogen atom because it is making four bonds. This systematic approach ensures that the structure accurately represents the ammonium salt.
 Created using AI
Created using AIWhat are the rules for naming asymmetrical ammonium salts?
When naming asymmetrical ammonium salts, follow these steps: First, identify the largest carbon chain connected to the nitrogen atom and name it as the parent chain with the suffix 'aminium.' Next, list other alkyl groups alphabetically as N-substituents. If there are identical alkyl groups, use numerical prefixes like di-, tri-, etc. For example, if you have an ammonium salt with a cyclopentane ring, an ethyl group, a methyl group, and an isopropyl group, the name would be 'N-ethyl-N-isopropyl-N-methylcyclopentanaminium.'
 Created using AI
Created using AIWhy is it important to know how to name and draw ammonium salts?
Knowing how to name and draw ammonium salts is crucial for understanding their properties and reactions in organic chemistry. Proper naming ensures clear communication among chemists and helps in identifying the structure and function of the compound. Drawing the correct structure is essential for predicting reactivity, understanding mechanisms, and designing experiments. This knowledge is foundational for advanced studies and practical applications in fields like pharmaceuticals, materials science, and chemical engineering.
 Created using AI
Created using AIWhat is the difference between ammonium ions and ammonium salts?
Ammonium ions (NH4+) are simple ions consisting of a nitrogen atom bonded to four hydrogen atoms, carrying a +1 charge. In contrast, ammonium salts are compounds where one or more alkyl groups replace some or all of the hydrogen atoms attached to the nitrogen, resulting in a positively charged nitrogen atom bonded to carbon atoms. The naming and structural conventions for ammonium salts are more complex due to the presence of these alkyl groups, whereas ammonium ions are straightforward and do not involve carbon atoms.
 Created using AI
Created using AIYour GOB Chemistry tutor
- Write the structures of the following compounds:a. Butyldiethylammonium bromide
- Write the structure of benzylamine hydrochloride in two different ways, and name the hydrochloride as an ammon...
- Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a pri...
- Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a pri...
- Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a pri...
- Draw the condensed structural or line-angle formula if cyclic, for each of the following: (14.5)d. ethylmethyl...
- Novocain, a local anesthetic, is the ammonium salt of procaine. (14.5)<IMAGE>a. Draw the condensed struc...
- Lidocaine (xylocaine) is used as a local anesthetic and cardiac depressant. (14.5) <IMAGE>...