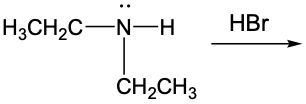
Amine reactions can be understood through the lens of acid-base chemistry, where an acid interacts with an amine, which acts as a weak base. In this context, when a base accepts a proton (H+), it is converted into its conjugate acid. For amines, this conjugate acid is referred to as the ammonium ion.
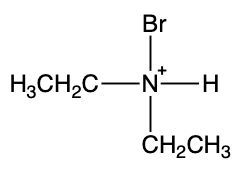
To illustrate this, consider the reaction between methylamine and hydrochloric acid. According to the Brønsted-Lowry definition, hydrochloric acid donates a proton to the methylamine. As methylamine accepts the H+ ion, the nitrogen atom forms four bonds, resulting in a positive charge. This transformation leads to the formation of the methylammonium ion, which is the ammonium ion derived from methylamine.
In summary, the reaction can be represented as follows:
Methylamine + HCl → Methylammonium ion + Cl-
This highlights the process of protonation of the amine, resulting in the creation of the corresponding ammonium ion, which is crucial in understanding the behavior of amines in acid-base reactions.