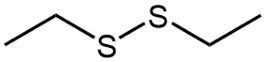
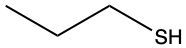
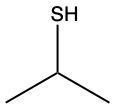
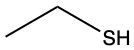
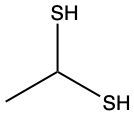
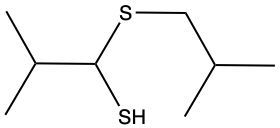
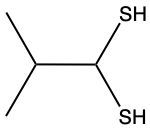
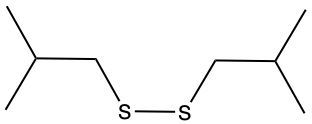
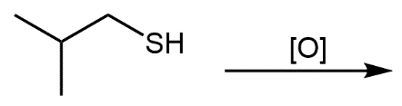The oxidation of thiols, which are organic compounds containing a sulfur-hydrogen (–SH) group, involves the reaction with an oxidizing agent such as bromine water (Br2 in water). In this process, two moles of thiol undergo oxidation to yield one mole of disulfide. The reaction can be summarized as follows: the –SH bonds in each thiol molecule are broken, leading to the formation of a new sulfur-sulfur (S–S) bond in the disulfide product.
From an oxidation perspective, this transformation involves the loss of hydrogen atoms. Specifically, each thiol loses one hydrogen, resulting in the two sulfur atoms bonding together to form the disulfide. This can be represented by the equation:
2 RSH → RSSR + H2
Here, R represents the organic group attached to the thiol. The process of oxidation is characterized by the gaining of oxygen or the loss of hydrogen, while reduction is defined as the addition of hydrogen. In the context of this reaction, reduction would involve breaking the S–S bond in the disulfide and adding back the lost hydrogens to regenerate the thiols:
RSSR + H2 → 2 RSH
In shorthand notation, oxidation can be represented as "O" and reduction as "H." Understanding these concepts is crucial for grasping the relationship between thiols and disulfides, as well as the broader principles of oxidation and reduction reactions in organic chemistry.