Here are the essential concepts you must grasp in order to answer the question correctly.
Ethers
Ethers are a class of organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups. They have the general formula R-O-R', where R and R' can be the same or different hydrocarbon chains. Ethers are known for their relatively low reactivity and are commonly used as solvents in organic chemistry.
Recommended video:
Nomenclature of Ethers
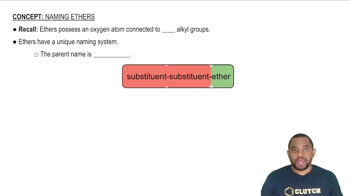
The nomenclature of ethers follows specific rules set by the International Union of Pure and Applied Chemistry (IUPAC). Ethers are typically named by identifying the two alkyl groups attached to the oxygen atom, followed by the word 'ether.' For example, the ether formed from ethyl and methyl groups is named ethyl methyl ether.
Recommended video:
Common Ethers
Some common examples of ethers include diethyl ether, which is often used as a solvent, and tetrahydrofuran (THF), a cyclic ether used in various chemical reactions. Recognizing these common ethers and their applications can aid in understanding their significance in both laboratory and industrial settings.
Recommended video: