Here are the essential concepts you must grasp in order to answer the question correctly.
Ethers
Ethers are a class of organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups. They have the general formula R-O-R', where R and R' represent the hydrocarbon chains. Ethers are known for their relatively low reactivity and are commonly used as solvents in organic chemistry due to their ability to dissolve a wide range of substances.
Recommended video:
Nomenclature of Ethers
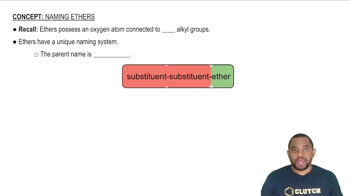
The nomenclature of ethers follows specific rules set by the International Union of Pure and Applied Chemistry (IUPAC). Ethers are typically named by identifying the two alkyl groups attached to the oxygen atom, followed by the word 'ether.' For example, in diethyl ether, the two ethyl groups are named first, indicating the structure of the compound.
Recommended video:
Common Ethers
Some common examples of ethers include diethyl ether, methyl tert-butyl ether (MTBE), and dimethyl ether. Each of these compounds has distinct properties and uses, such as diethyl ether being a well-known solvent and anesthetic, while MTBE is often used as a fuel additive. Recognizing these common ethers aids in understanding their applications and significance in both laboratory and industrial settings.
Recommended video:
 Verified step by step guidance
Verified step by step guidance