Here are the essential concepts you must grasp in order to answer the question correctly.
Amine Structure
Amines are organic compounds derived from ammonia (NH3) by replacing one or more hydrogen atoms with alkyl or aryl groups. The general structure of an amine can be represented as R-NH2, R2-NH, or R3-N, where R represents an alkyl or aryl group. Understanding the basic structure of amines is essential for drawing their chemical representations.
Recommended video:
Triethylamine
Triethylamine is a specific type of tertiary amine with the chemical formula N(CH2CH3)3. It consists of three ethyl groups attached to a nitrogen atom, which does not have any hydrogen atoms directly bonded to it. Recognizing the structure of triethylamine is crucial for accurately drawing its molecular representation.
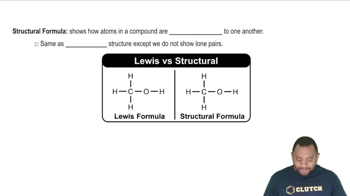
Drawing Chemical Structures
Drawing chemical structures involves representing the arrangement of atoms and bonds in a molecule. This includes using lines to depict bonds between atoms and ensuring that the valency of each atom is satisfied. Mastery of drawing techniques is important for visualizing and communicating the structure of compounds like triethylamine.
Recommended video:
 Verified step by step guidance
Verified step by step guidance