Now the first ionization energy, abbreviated as IE1, is the energy absorbed to remove the first electron from a gaseous atom. So remember we're moving. The electron makes nitrogen now positively charged. It's still in its gaseous phase and here is the electron we removed that has associated with this ionization energy of 1402, which will be in kilojoules now.
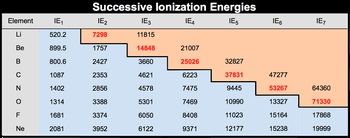
With more energy you can remove additional electrons in successive ionizations. So you can remove the 2nd electron from Na+ to give us ionization energy 2. You could try to remove the third one, which would require ionization energy 3 and so on. Now successive ionizations is the removing of additional electrons in stages instead of all at once.
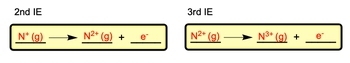
If I'm trying to remove 3 electrons from nitrogen, I can't move them all at the same time, I have to remove them one by one. So here we have the first ionization energy of nitrogen. So if I was trying to remove the next electron, I've already removed the first. So now I'm at this plus one stage, I'm removing the second one so now it becomes 2+ and here's the electron I removed.
So remember as I remove each electron the ion becomes more positively charged. Then I need to remove the third electron. I've already moved the first two, so at present I'm N2+. I'm about to remove the third electron and when I do that I become 3+ and here goes the electron I just removed. So this would be ionization energy one, ionization energy two, and then ionization energy 3 where we remove the electron in three separate stages.