Recall that a heteronuclear diatomic molecule is composed of two different elements bonded together, and we're going to say here that the less electronegative element determines which molecular orbital will be used. And recall that electronegativity increases as you move to the top right corner of the periodic table. Remember, fluorine is the most electronegative element with a value of 4.0.
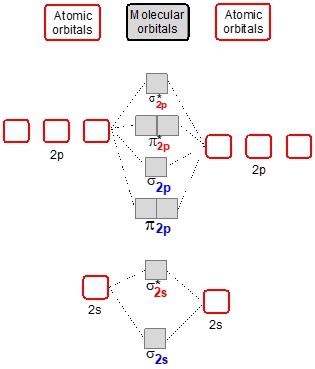
Now we're going to say here that when it comes to displaying these molecular orbitals or these molecular orbital diagrams, the more electronegative element within the heteronuclear diatomic molecule possesses atomic orbitals that are lower in energy. So here, if we take a look, we have molecular orbital diagrams for hydrogen to helium, for lithium to nitrogen, and from oxygen to neon. If you've watched my videos on molecular orbital theory, you're kind of familiar with these displays.
Now if you notice, you can see here that this 2S atomic orbital is higher in energy than this one. This one is lower down. That must mean that let's say we're combining. We're comparing carbon to nitrogen. Carbon is less electronegative than nitrogen. Since nitrogen is more electronegative, it would be here on this side and carbon would be here on this side. And since nitrogen is more electronegative, that explains why its atomic orbitals are lower in energy.
See here. It's 2P orbitals are also lower in energy, carbons are higher in energy. And then here if we look at oxygen to neon, same thing. This 2S orbital atomic orbital is higher in energy than this one, meaning the left side is involved with an element that has less electronegative. So for example, maybe we have here oxygen versus fluorine.
OK, so keep in mind that electronegativity helps us determine which molecular orbital diagram to use and also it helps us to see the differences in energy for our atomic orbitals. Now just remember that the higher your electronegativity than the electrons will be closer to the nucleus, so they're going more tightly bound to the nucleus. What effect can this have on our shell number here?
So here our electrons are being pulled closer and closer to the nucleus. Since electronegativity is increasing, this will see a lot of the times with lower numbered shell elements such as flooring. So here we have a lower shell number. All right, so keep in mind these fundamental ideas when we're talking about heteronuclear diatomic molecules and MO theory.