Here we're told that a gaseous compound of nitrogen and hydrogen is found to have a density of OH .977g per liter at .69474 atmospheres and 373.15 Kelvin. What is the molecular formula of the compound? Well, realize here in this question they're giving us density, they're giving us pressure, and they're giving us temperature. With this information, I could find the molar mass of this unknown gas.
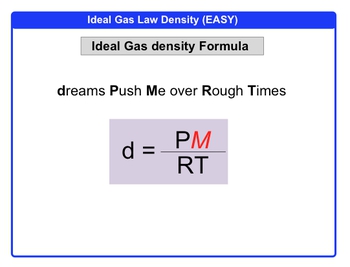
So here we're going to say that dreams push me over rough times, and we're going to say that we have density, we have pressure, we have temperature, and we always know what R is. We just need to isolate our molar mass. So multiply both sides by RT, so RT times D = P times molar mass. Divide both sides by pressure so molar mass equals
D
R
T
P
Or take the information given to us. Our density is OH .977g per liter. R is our gas constant. Temperature is already in Kelvin and pressure is already in atmospheres. Doing this we'll see that we isolate. So atmospheres are gone, kelvins are gone, liters are gone, we'll have grams per mole.
So when we plug this into our formula, we're going to get here as our mass, 43.06g per mole. So here's our molar mass. All we do now is we look at the different compounds and we would just calculate their molar masses and see which one comes closest to this 43.06g per mole. Now if you did this correctly, you would see that the answer would have to be option C.
It's the one with the mask closest to our answer. It has a molar mass approximately equal to 43.038g per mole. So option C would be the answer for this particular question. So realize you're giving us density, pressure, and temperature. That means we can use the density form of the ideal gas law to help us find molar mass and then use that information to compare to the molar masses of all these gases.