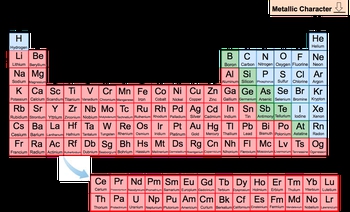
Now before we can talk about metallic characteristics of the elements in the periodic table, it's first important to talk about the periodic trends themselves. Now, the periodic trends are specific patterns in the property of elements based on their changing atomic numbers.
We're going to say as we examine these periodic trends, we will examine these patterns while moving to the top right corner of the periodic table. So we're generally going to be moving this way to the top right corner of the periodic table when discussing the different types of periodic trends that exist.
O keep that in mind as we investigate each and everyone.