The four factors that have a direct impact on influencing reaction rates include concentration of reactants, surface area of reactants, temperature, and catalysts. Now here if we take a look at concentration of reactants, first we're going to say the condition is if we increase the reactant concentrations, what effect does this have on collisions? Well, increasing the amount of reactants within a mixture means they're going to be more reactants floating around. This is going to result in an increase in collision frequency because since there's more molecules floating around, you have a higher likelihood of them bouncing into each other. This results in an increase in your rate.
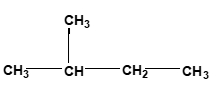
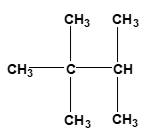
Now surface area of reactants. Here you want to have a larger surface area, so we want to increase our surface area. Usually when we talk about surface area for compounds, we can look at compounds as either being linear or branched. When we say linear, linear compounds have higher surface areas. Here we have hydrocarbon. It's made up of CH3, CH2, CH2, CH3. Branched means that whereas in linear we just have a straight line of elements together, something that's branched has a portion of it that's sticking out. Here, this CH3 part is a branching group. Branching decreases our surface area. So just remember, if we can increase the surface area of our reactants, that's going to cause an increase in our collision frequency, which in itself again will cause an increase in our rate.
Now temperature, we're going to say here that increasing, increasing our temperature is going to cause an increase in our collision frequencies. If you think of it in terms of your heating up, your mixture, your solution which has reacted molecules floating around, if you're heating it up, those molecules will absorb that extra external heat source and basically use it to power themselves up. This will cause them to move around with greater energy. If they're moving faster, they can bump into each other even faster. So this is going to cause an increase in collision frequency because there's an increase in collision energy from absorbing the thermal energy. Now we're going to say the rule of thumb is if we increase the temperature by just 10°C, this causes a doubling of our rate, so our rate would increase by 2X. So increasing temperature increases collisions, increases the force of the collisions because the molecules are moving faster. All of this causes an increase in our rate.
Finally, a catalyst, we're going to say the addition of a catalyst. Remember, addition of a catalyst causes a decrease in your activation energy. And that's a good thing, because the lower your activation energy is, the more likely that your reactants can become products. So decreasing our activation energy will cause an increase in successful collisions, which in turn will cause an increase in your rate. So these are the four factors you need to keep in mind when dealing with reaction rates. Affecting them can have a big impact on how fast or slow your chemical reaction will proceed.