Here are the essential concepts you must grasp in order to answer the question correctly.
Amines
Amines are organic compounds derived from ammonia (NH3) by replacing one or more hydrogen atoms with alkyl or aryl groups. They can be classified as primary, secondary, or tertiary based on the number of carbon-containing groups attached to the nitrogen atom. Amines are characterized by their basicity and can participate in hydrogen bonding, influencing their physical properties.
Recommended video:
Isopropyl Group
The isopropyl group is a branched alkyl group with the formula -CH(CH3)2, derived from propane. It consists of a central carbon atom bonded to two methyl groups and one hydrogen atom. This structure is significant in organic chemistry as it affects the reactivity and properties of the compounds it is part of, such as isopropylamine.
Recommended video:
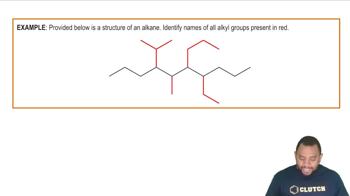
Structural Representation
Structural representation in chemistry involves depicting the arrangement of atoms within a molecule. This can be done using various methods, such as Lewis structures, condensed formulas, or skeletal formulas. Understanding how to draw these structures is essential for visualizing molecular geometry, predicting reactivity, and communicating chemical information effectively.
Recommended video:
 Verified step by step guidance
Verified step by step guidance