Here are the essential concepts you must grasp in order to answer the question correctly.
CO2 Emissions and Ocean Acidification
Increased CO2 emissions lead to higher concentrations of carbon dioxide in the atmosphere, which subsequently dissolves in ocean water, forming carbonic acid. This process lowers the pH of seawater, resulting in ocean acidification. Ocean acidification negatively impacts marine organisms, particularly those that rely on calcium carbonate for their shells and skeletons, such as corals and mollusks.
Recommended video:
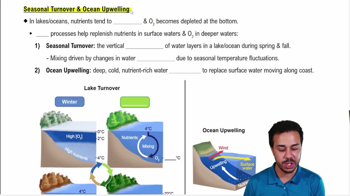
Seasonal Turnover & Ocean Upwelling
Net Ecosystem Calcification
Net ecosystem calcification refers to the balance between the processes of calcification (the production of calcium carbonate by organisms) and dissolution (the breakdown of calcium carbonate). In marine ecosystems, this balance is crucial for maintaining healthy coral reefs and other calcifying organisms. Changes in environmental conditions, such as pH and carbonate ion concentration, can significantly affect this balance.
Recommended video:
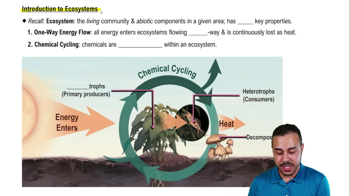
Introduction to Ecosystems
Experimental Variables
In scientific experiments, variables are the factors that can be changed or controlled to test their effects on the outcome. In the context of this study, variables likely altered include CO2 concentration, temperature, and nutrient levels in the seawater tanks. By manipulating these variables, researchers can simulate future ocean conditions and assess their impact on net ecosystem calcification.
Recommended video:

 Verified step by step guidance
Verified step by step guidance