Back
BackProblem 1
Which of the following occurs when a covalent bond forms?
a. Electrons in valence shells are transferred from one atom to another.
b. Electrons in valence shells are shared between atoms.
c. Partial charges on polar molecules interact.
Problem 3
Which of these functional groups is known to be used for storing large amounts of chemical energy? a. amino group b. carbonyl group c. phosphate group d. sulfhydryl group
Problem 5
Which of these molecules would you predict to have the largest number of polar covalent bonds based on their molecular formulas? a. C2H6O (ethanol) b. C2H6 (ethane) c. C2H4O2 (acetic acid) d. C3H8O (propanol)
Problem 6
Locate fluorine (F) on the partial periodic table provided in Figure 2.2. Predict its relative electronegativity compared to hydrogen, sodium, and oxygen. State the number and type of bond(s) you expect it would form if it reacted with sodium (Na).

Problem 7
If you were given a solution that has a pH of 8.5, what would be its concentration of protons? What is the difference in proton concentration between this solution and one that has a pH of 7?
Problem 8
Consider the reaction between carbon dioxide and water to form carbonic acid: CO2(g)+H2O(l)⇌CH2O3(aq) In the ocean, carbonic acid immediately dissociates to form a proton and bicarbonate ion, as follows: CH2O3(aq)+H+(aq)⇌CHO3−(aq) If an underwater volcano bubbled additional CO2 into the ocean, would this sequence of reactions be driven to the left or the right? How would this affect the pH of the ocean?
Problem 9
When H2 and CO2 react, acetic acid can be formed spontaneously while the production of formaldehyde requires an input of energy. Which of these conclusions can be drawn from this observation? a. More heat is released when formaldehyde is produced compared to the production of acetic acid. b.Compared to the reactants that it is formed from, formaldehyde has more potential energy than does acetic acid. c. Entropy decreases when acetic acid is produced and increases when formaldehyde is produced. d. Only acetic acid could be produced under conditions that existed in early Earth.
Problem 10
From what you have learned about water, why do coastal regions tend to have milder climates with cooler summers and warmer winters than do inland areas at the same latitude?
Problem 11
Consider the reaction between carbon dioxide and water to form carbonic acid (CH2O3):
CO2(𝑔)+H2O(𝑙)⇌CH2O3(𝑎𝑞)
In the ocean, carbonic acid immediately dissociates to form a proton and bicarbonate ion, as follows:
CH2O3(𝑎𝑞)⇌CHO3−(𝑎𝑞)+H+(𝑎𝑞)
As atmospheric CO2 increases, the ocean absorbs more of the gas. Would this sequence of reactions be driven to the left or the right? How would this affect the pH of the ocean?
Problem 12
The current average pH of our oceans is 8.1. What is the concentration of protons in the oceans? How has the proton concentration changed in our oceans when compared to before the industrial revolution, when the average pH was 8.2? Express this change as a percentage increase.
Problem 13
Stony corals secrete thin layers of calcium carbonate (CaCO3) to build the foundation of coral reefs. The relationship between calcium carbonate, carbonic acid, and calcium bicarbonate (Ca(HCO3)2) is shown below:
CH2O3(𝑎𝑞)+CaCO3(𝑠) ⇌ Ca(HCO3)2(𝑎𝑞)
Predict what will happen to the calcium carbonate foundation of reefs as CO2 levels rise in the oceans.
Problem 15
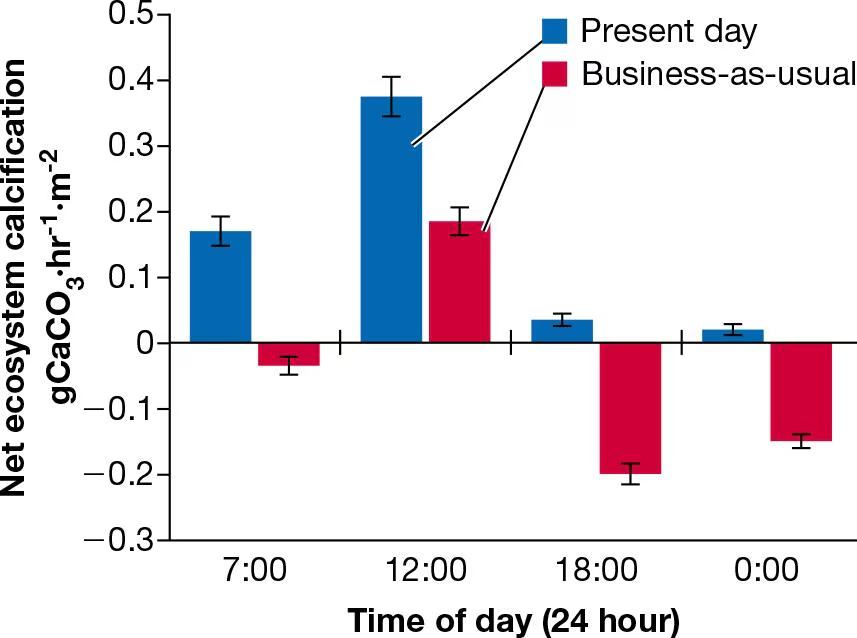
Data from the preceding experiment were collected at different times throughout each day over a period of one year under both present-day and estimated year 2100 conditions. Averages from these samples are provided in the following graph
Using the equation in Question 13, what do the positive and negative values indicate in terms of the directionality of this reaction? What implications do these data have on reef stability in the year 2100 if there is no intervention?