From what you have learned about water, why do coastal regions tend to have milder climates with cooler summers and warmer winters than do inland areas at the same latitude?
Stony corals secrete thin layers of calcium carbonate (CaCO3) to build the foundation of coral reefs. The relationship between calcium carbonate, carbonic acid, and calcium bicarbonate (Ca(HCO3)2) is shown below:
CH2O3(𝑎𝑞)+CaCO3(𝑠) ⇌ Ca(HCO3)2(𝑎𝑞)
Predict what will happen to the calcium carbonate foundation of reefs as CO2 levels rise in the oceans.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Calcium Carbonate Chemistry

Ocean Acidification
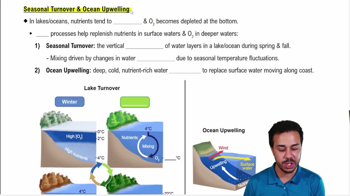
Coral Reef Ecosystem Dynamics

Consider the reaction between carbon dioxide and water to form carbonic acid (CH2O3):
CO2(𝑔)+H2O(𝑙)⇌CH2O3(𝑎𝑞)
In the ocean, carbonic acid immediately dissociates to form a proton and bicarbonate ion, as follows:
CH2O3(𝑎𝑞)⇌CHO3−(𝑎𝑞)+H+(𝑎𝑞)
As atmospheric CO2 increases, the ocean absorbs more of the gas. Would this sequence of reactions be driven to the left or the right? How would this affect the pH of the ocean?
The current average pH of our oceans is 8.1. What is the concentration of protons in the oceans? How has the proton concentration changed in our oceans when compared to before the industrial revolution, when the average pH was 8.2? Express this change as a percentage increase.
Data from the preceding experiment were collected at different times throughout each day over a period of one year under both present-day and estimated year 2100 conditions. Averages from these samples are provided in the following graph
Using the equation in Question 13, what do the positive and negative values indicate in terms of the directionality of this reaction? What implications do these data have on reef stability in the year 2100 if there is no intervention?
