Here are the essential concepts you must grasp in order to answer the question correctly.
Work Output (Wₒᵤₜ)
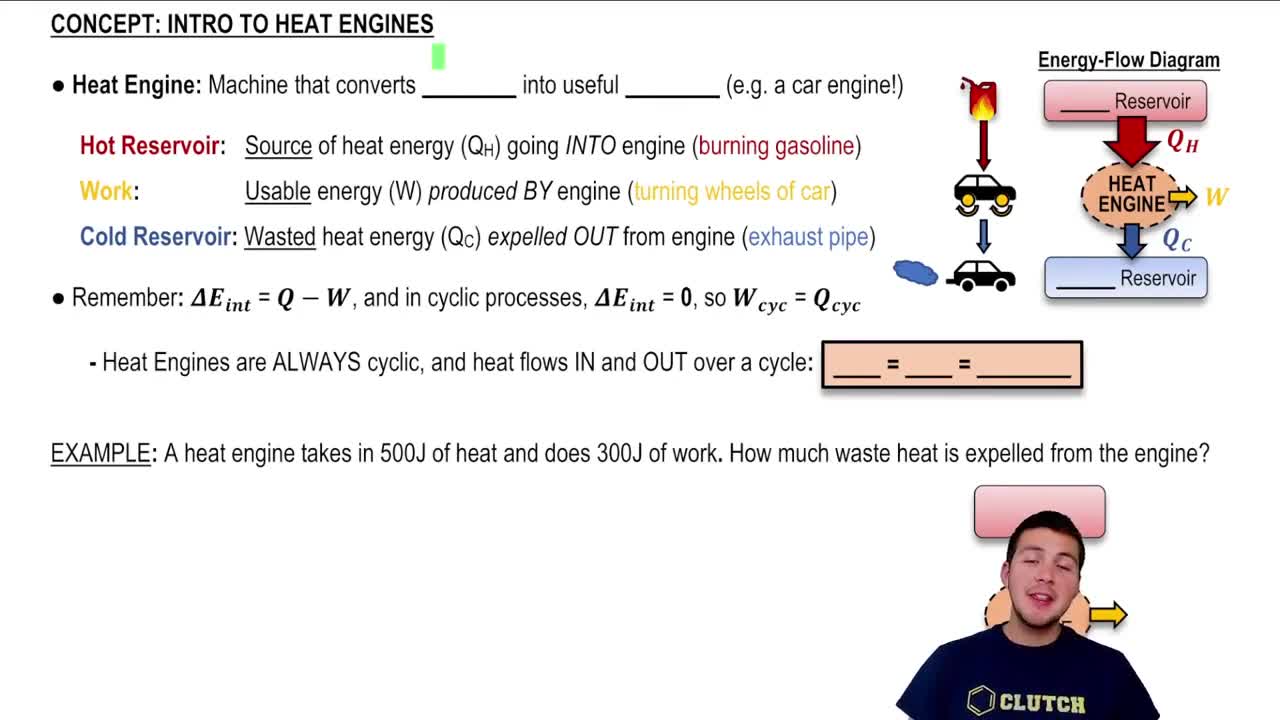
Work output (Wₒᵤₜ) refers to the useful work produced by a heat engine during its operation. It is the energy converted from thermal energy into mechanical energy, typically measured in joules. This value is crucial for determining the performance of the engine, as it indicates how effectively the engine converts heat into work.
Recommended video:
Power Output of a Gasoline Engine
Heat Input (QH)
Heat input (QH) is the total amount of thermal energy supplied to the heat engine from a high-temperature source. This energy is essential for the engine's operation, as it provides the necessary heat to drive the thermodynamic cycle. Understanding QH is vital for calculating the engine's efficiency and performance.
Recommended video:
Introduction to Heat Engines
Thermal Efficiency
Thermal efficiency is a measure of how effectively a heat engine converts heat input into work output. It is defined as the ratio of work output (Wₒᵤₜ) to heat input (QH), often expressed as a percentage. Higher thermal efficiency indicates a more effective engine, minimizing wasted energy and maximizing performance.
Recommended video:
Thermal Efficiency & The Second Law of Thermodynamics