Here are the essential concepts you must grasp in order to answer the question correctly.
Thermal Efficiency
Thermal efficiency is a measure of how effectively a heat engine converts heat energy into work. It is defined as the ratio of the work output to the heat input, expressed as a percentage. The formula for thermal efficiency (η) is η = (Work Output / Heat Input) × 100%. In this case, it helps determine how well the engine utilizes the energy it receives.
Recommended video:
Thermal Efficiency & The Second Law of Thermodynamics
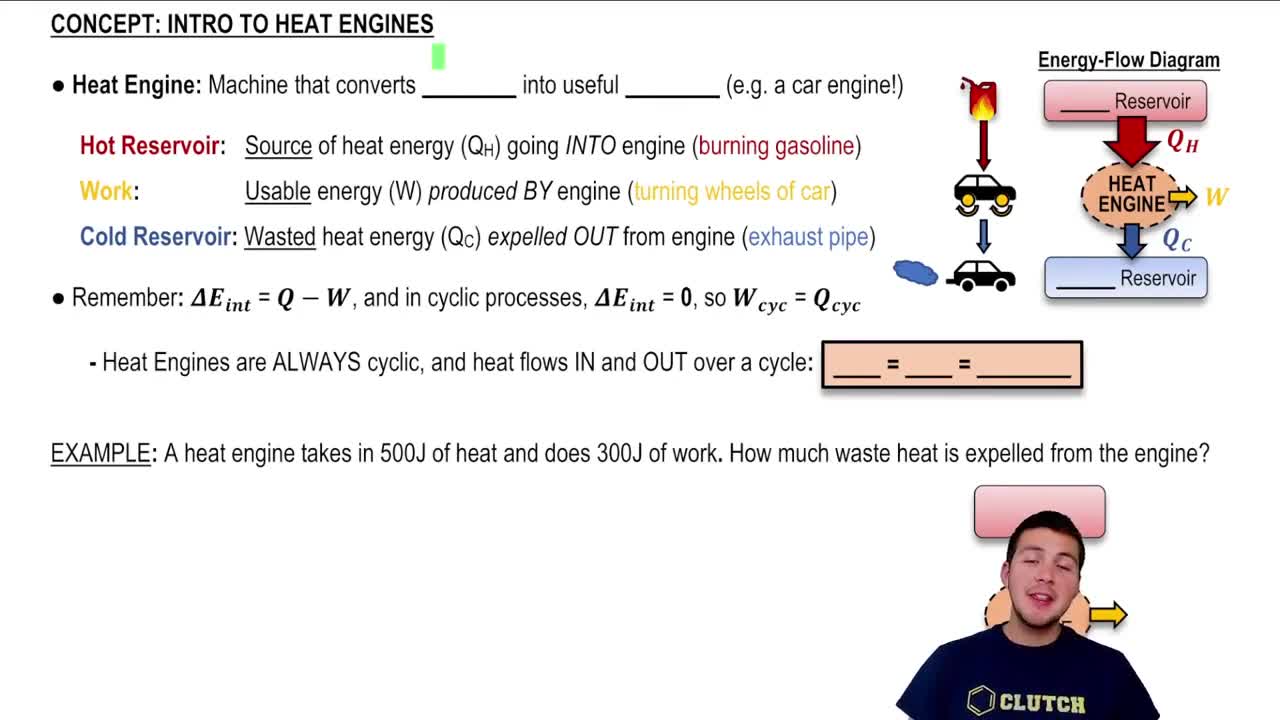
First Law of Thermodynamics
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another. In the context of a heat engine, this principle implies that the total energy input (heat) must equal the sum of the work done by the engine and the waste heat expelled. This law is fundamental in analyzing energy transfers in thermodynamic systems.
Recommended video:
The First Law of Thermodynamics
Heat Engine Cycle
A heat engine cycle refers to the series of processes that a heat engine undergoes to convert heat energy into mechanical work. Typically, this cycle includes phases of heat absorption, work output, and heat rejection. Understanding the cycle is crucial for calculating efficiency, as it outlines how energy is transformed and where losses occur, such as in the waste heat.
Recommended video:
Introduction to Heat Engines