Here are the essential concepts you must grasp in order to answer the question correctly.
Power and Work
Power is the rate at which work is done or energy is transferred over time. In the context of engines, power output can be calculated using the formula P = W/t, where P is power, W is work, and t is time. Understanding this relationship is crucial for determining the total work done by the engine during its operation.
Recommended video:
Thermal Efficiency
Thermal efficiency is a measure of how effectively an engine converts the energy from fuel into useful work. It is defined as the ratio of the work output to the heat input, expressed as a percentage. In this case, with a thermal efficiency of 20%, only 20% of the energy input is converted into work, while the rest is lost as heat.
Recommended video:
Thermal Efficiency & The Second Law of Thermodynamics
Heat Exhaust
Heat exhaust refers to the thermal energy that is not converted into work and is expelled from the engine. It can be calculated by determining the total energy input and subtracting the useful work output. This concept is essential for understanding energy losses in engines and the overall performance of thermal systems.
Recommended video:
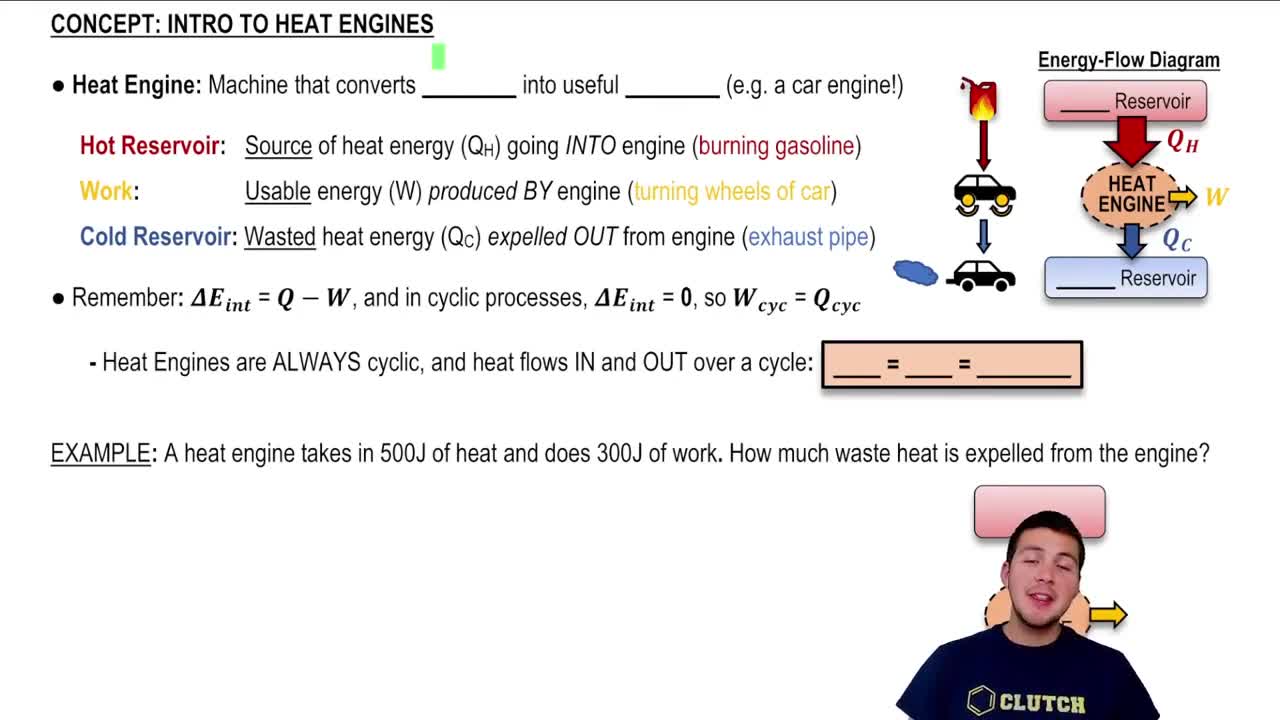
Introduction to Heat Engines
 Verified step by step guidance
Verified step by step guidance