Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is fundamental for understanding gas behavior under varying conditions. In this scenario, it helps determine how temperature changes when volume and pressure are manipulated.
Recommended video:
Ideal Gases and the Ideal Gas Law
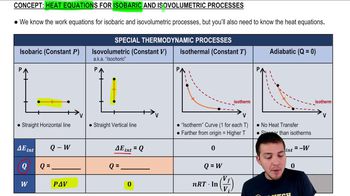
Isobaric Process
An isobaric process is a thermodynamic process in which the pressure remains constant while the volume and temperature of the gas change. During this type of expansion, the gas absorbs heat, leading to an increase in temperature as the volume increases. Understanding this concept is crucial for calculating the final temperature after the gas expands.
Recommended video:
Heat Equations for Isobaric & Isovolumetric Processes
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is held constant. This principle is essential for determining the final temperature of the gas after it has expanded isobarically. It allows us to relate the initial and final states of the gas to find the new temperature after the volume has tripled.
Recommended video: