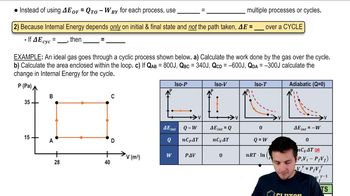
Here are the essential concepts you must grasp in order to answer the question correctly.
Isochoric Process
An isochoric process is a thermodynamic process in which the volume of the system remains constant. During this process, any change in temperature or pressure occurs without any work being done on or by the system, as work is defined as force applied over a distance. This means that the internal energy of the gas changes solely due to heat transfer.
Recommended video:
Properties of Cyclic Thermodynamic Processes
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in thermodynamics that relates the pressure (p), volume (V), and temperature (T) of an ideal gas. It is expressed as PV = nRT, where n is the number of moles and R is the ideal gas constant. This law allows us to understand how changes in one state variable affect the others, particularly useful in analyzing processes like the one described.
Recommended video:
Ideal Gases and the Ideal Gas Law
Pressure-Temperature Relationship
In an isochoric process, the pressure of a gas is directly proportional to its temperature, as described by the Ideal Gas Law. When the volume is held constant, cooling the gas (decreasing temperature) results in a decrease in pressure. This relationship is crucial for determining the final state variables of the gas after the cooling process, as indicated by the condition p₂ = 1/3p₁.
Recommended video:
Doubling Pressure & Temperature