23. The Second Law of Thermodynamics
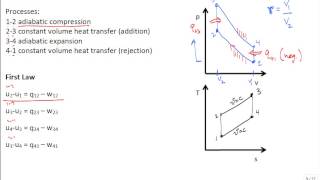
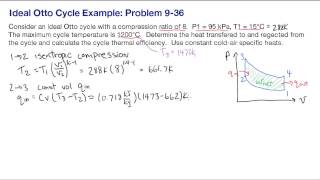
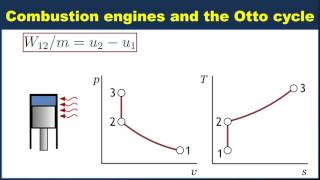
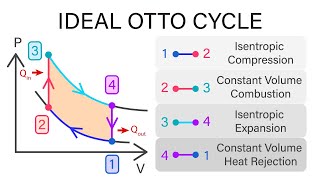
The Otto Cycle
Learn with other creators
Practice this topic
- Textbook Question
The Otto-cycle engine in a Mercedes-Benz SLK230 has a compression ratio of . What is the ideal efficiency of the engine? Use .
1884views - Textbook Question
Calculate the theoretical efficiency for an Otto-cycle engine with and .
1672views - Textbook Question
A car's internal combustion engine can be modeled as a heat engine operating between a combustion temperature of 1500℃ and an air temperature of 20℃ with 30% of the Carnot efficiency. The heat of combustion of gasoline is 47 kJ/g. What mass of gasoline is burned to accelerate a 1500 kg car from rest to a speed of 30 m/s?
1312views - Textbook Question
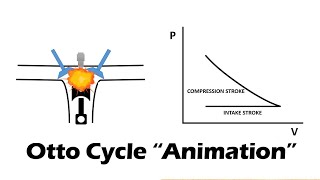
The gasoline engine in your car can be modeled as the Otto cycle shown in FIGURE CP21.73. A fuel-air mixture is sprayed into the cylinder at point 1, where the piston is at its farthest distance from the spark plug. This mixture is compressed as the piston moves toward the spark plug during the adiabatic compression stroke. The spark plug fires at point 2, releasing heat energy that had been stored in the gasoline. The fuel burns so quickly that the piston doesn't have time to move, so the heating is an isochoric process. The hot, high-pressure gas then pushes the piston outward during the power stroke. Finally, an exhaust value opens to allow the gas temperature and pressure to drop back to their initial values before starting the cycle over again. Analyze the Otto cycle and show that the work done per cycle is
920views