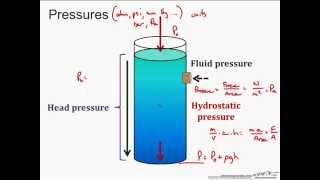
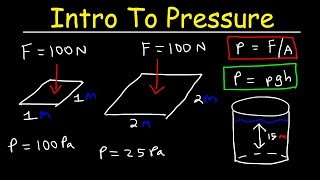
19. Fluid Mechanics
Intro to Pressure
Learn with other creators
Practice this topic
- Multiple Choice
A large warehouse is 100 m wide, 100 m deep, 10 m high:
a) What is the total weight of the air inside the warehouse?
b) How much pressure does the weight of the air apply on the floor?1577views14rank2comments - Multiple Choice
A tall cylindrical beaker 10 cm in radius is placed on a picnic table outside. You pour 5 L of an 8,000 kg/m3 liquid and 10 L of a 6,000 kg/m3 liquid into. Calculate the total pressure at the bottom of the beaker. (Use g=10 m/s2.)
1326views4rank2comments - Multiple Choice
A wooden cube, 1 m on all sides and having density 800 kg/m3 , is held under water in a large container by a string, as shown below. The top of the cube is exactly 2 m below the water line. Calculate the difference between the force applied by water to the top and to the bottom faces of the cube (Hint:calculate the two forces, then subtract.Use g=10 m/s2.)
1565views14rank5comments - Multiple ChoiceAt point A, a hose ejects water at a speed of . Point B is upstream from, and 30.0cm above, the outlet. If the water is moving at at point B, what is the internal pressure of the water at B? Use for atmospheric pressure and for the density of water.1256views
- Textbook Question
BIO. The lower end of a long plastic straw is immersed below the surface of the water in a plastic cup. An average person sucking on the upper end of the straw can pull water into the straw to a vertical height of 1.1 m above the surface of the water in the cup. (a) What is the lowest gauge pressure that the average person can achieve inside his lungs? (b) Explain why your answer in part (a) is negative.
2331views - Textbook Question
Exploring Venus. The surface pressure on Venus is 92 atm, and the acceleration due to gravity there is 0.894g. In a future exploratory mission, an upright cylindrical tank of benzene is sealed at the top but still pressurized at 92 atm just above the benzene. The tank has a diameter of 1.72 m, and the benzene column is 11.50 m tall. Ignore any effects due to the very high temperature on Venus. What force does the Venusian atmosphere exert on the outside surface of the bottom of the tank?
1850views - Textbook Question
A closed container is partially filled with water. Initially, the air above the water is at atmospheric pressure (1.01×105 Pa) and the gauge pressure at the bottom of the water is 2500 Pa. Then additional air is pumped in, increasing the pressure of the air above the water by 1500 Pa. What is the gauge pressure at the bottom of the water?
2379views - Textbook Question
A barrel contains a 0.120-m layer of oil floating on water that is 0.250 m deep. The density of the oil is 600 kg/m3. (a) What is the gauge pressure at the oil–water interface? (b) What is the gauge pressure at the bottom of the barrel?
2125views