Here are the essential concepts you must grasp in order to answer the question correctly.
Definition of a Chemical Element
A chemical element is a pure substance that cannot be broken down into simpler substances by chemical means. Each element is defined by the number of protons in its atomic nucleus, known as the atomic number. Elements are the building blocks of matter and are represented in the periodic table.
Recommended video:
Atomic Structure
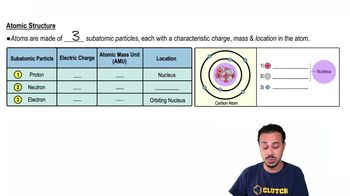
The atomic structure of a chemical element consists of protons, neutrons, and electrons. Protons and neutrons reside in the nucleus, while electrons orbit around the nucleus in defined energy levels. The arrangement and number of these subatomic particles determine the element's properties and its behavior in chemical reactions.
Recommended video:
Periodic Table
The periodic table is a systematic arrangement of chemical elements, organized by increasing atomic number and grouped by similar chemical properties. It provides essential information about each element, including its symbol, atomic mass, and electron configuration. Understanding the periodic table is crucial for predicting how elements will interact in chemical reactions.
Recommended video:
Review Table of Immunoglobin Classes