Now recall that stoichiometry deals with the numerical relationship between compounds in a balanced chemical equation. Now with a thermochemical equation, we're going to deal with chemical reactions that include an enthalpy of reaction, which is ΔHrxn, so delta H of reaction. Here with thermochemical equations, we're going to be introduced to our thermochemical stoichiometric chart. Now here, the chart uses the given quantity of a compound to determine the unknown quantity of another compound. So here with the thermochemical equation, we have our balanced chemical equation, and to the side of it, you'll see your delta H of reaction. It's our job to make a connection between your enthalpy of reaction and either moles, grams, molecules, what have you, in terms of the chemical reaction. So in a thermochemical equation, what we're trying to do is not a mole-to-mole comparison; we're trying to do a delta H to mole comparison. And that's the key difference with our thermochemical equation. Now that we've seen this, let's move onwards and talk about more, with thermochemical equations.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Thermochemical Equations: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIStoichiometry involves the numerical relationships in balanced chemical equations, while thermochemical equations incorporate the enthalpy of reaction (ΔHrxn). In these equations, the focus shifts from mole-to-mole comparisons to ΔH-to-mole comparisons. By using a thermochemical stoichiometric chart, one can convert given ΔH to moles of a compound, then make a mole-to-mole comparison using coefficients from the balanced equation to find unknown quantities. This process highlights the connection between enthalpy and various forms of measurement, such as grams or molecules, essential for understanding thermochemistry.
Thermochemical Equations involve a balanced chemical equation with a given enthalpy value.
Thermochemical Equations
Thermochemical Equations
Video transcript
Thermochemical Equations
Video transcript
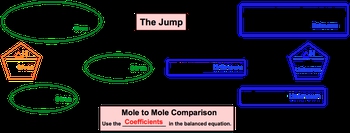
Now, if you've seen my stoichiometric chart under stoichiometry, or solution chemistry, or gas stoichiometry, this should be pretty familiar to you. But if this is the first time you're seeing this stoichiometric chart, let's go through it. Alright. So what we need to realize here is that with thermochemical equations, it's pretty common to be given the ΔH of our chemical reaction. So we'll start out with ΔH of given. And like I said before, in stoichiometry we're used to doing a multiple comparison which can still happen here, but the more important thing is that we establish a connection between the ΔH of reaction and one of the moles for one of the compounds within our chemical, reaction. So, here we go from ΔH of given to moles of given. And what we can say here is that besides going from ΔH of given to moles of given, we can go from grams of given to moles of given, or we can go from ions, atoms, formula units, or molecules of given to moles of given. Once we get there, we have to go to moles of our unknown. To do this requires a leap of faith in a sense, because you're going from an area where you know information to an area where you know nothing at all. So, we call this the jump. When you make this jump, in order to do it correctly you have to do a mole to mole comparison and use the coefficients in the balanced equation. From this point, you are at moles of unknown, and from here you can go in any way you want. You can go from moles of unknown to ions, atoms, formula units, or molecules, you can go to grams, or you can go to a new ΔH of unknown. So, just realize with the thermochemical equation, we'll have a balanced chemical equation, which introduces the idea of stoichiometry, but for it to be a thermochemical equation, we'll also have the ΔH of reaction present.
A modified version of the stoichiometric chart can used for thermochemical equations.
Thermochemical Equations Example 1
Video transcript
Here it says, consider the following thermochemical reaction. We have 2 moles of magnesium solid reacting with 1 mole of oxygen gas to produce 2 moles of magnesium oxide solid. It gives us an enthalpy of reaction equal to negative 1204 kilojoules. Here we're asked how many grams of magnesium oxide are produced during enthalpy change of negative 375 kilojoules. So what we have to do here is we have to convert the given quantity into moles of given. Alright. So they're giving us negative 375 kilojoules of energy, and what we need to do is establish a relationship between magnesium oxide and this value of negative 375. Well according to my balanced equation, for step 2, it says we need to do a mole to mole comparison to convert moles of given into moles of unknown. Here because it's a thermochemical equation, it's going to be moles of given relating to delta h of reaction. So we're going to say for every 2 moles of magnesium oxide, the energy involved or enthalpy involved is negative 1204 kilojoules per mole. So we've just found our moles in terms of magnesium oxide.
Steps 3 says, if necessary convert the moles into desired units. Here they want grams not moles, so we're going to do one more step and say for every one mole of magnesium oxide, the mass of magnesium oxide, 1 magnesium, is 24.31 grams according to the periodic table. One oxygen is 16 grams. Multiplying and adding those numbers together gives us 40.31 grams. Moles cancel out and now I'm going to have 25.11 grams of magnesium oxide. Step 4 isn't needed here because, in step 4, if we have to calculate more than one final amount then we must compare them to determine the theoretical yield. Here we're only given one given the amount of negative 375 kilojoules and using that helped us to determine the final answer of 25.11 magnesium oxide, grams of magnesium oxide.
Nitromethane (CH3NO2), sometimes used as a fuel for drag racing, burns according to the following reaction:
4 CH3NO2 (l) + 7 O2 (g) → 4 CO2 (g) + 6 H2O (g) + 4 NO2 (g) ∆Hº = – 2441.6 kJ
How much heat is released by burning 125.0 g of nitromethane (MW:61.044 g/mol)?
Consider the following reaction:
2 C6H6 (l) + 15 O2 (g) → 12 CO2 (g) + 6 H2O (g) ∆Hº = – 6278 kJ
What volume of benzene (C6H6, d = 0.880 g/mL, molar mass = 78.11 g/mol) is necessary to evolve 5.19 x 109 kJ of heat?
1.47 x 108 mL
5.19 x 109 mL
4.37 x 107 mL
9.51 x 109 mL
The creation of liquid methanol is accomplished by the hydrogenation of carbon monoxide:
CO (g) + 2 H2 (g) → CH3OH (l) ∆Hº = – 128.1 kJ
How much heat (in kJ) is released when 125.0 g CO reacts with 2.32 x 102 g H2?
Here’s what students ask on this topic:
What is a thermochemical equation?
A thermochemical equation is a balanced chemical equation that includes the enthalpy change (ΔH) of the reaction. This ΔH value represents the heat absorbed or released during the reaction. Unlike standard stoichiometric equations that focus on mole-to-mole comparisons, thermochemical equations emphasize the relationship between ΔH and the moles of reactants or products. For example, in the combustion of methane: CH4 + 2O2 → CO2 + 2H2O, ΔH = -890 kJ, the ΔH value indicates that 890 kJ of energy is released per mole of CH4 combusted.
 Created using AI
Created using AIHow do you use a thermochemical stoichiometric chart?
A thermochemical stoichiometric chart helps convert given quantities (like ΔH, grams, or molecules) to moles and then to other desired quantities. Start with the given ΔH and convert it to moles of the given substance. From there, use the balanced chemical equation to perform a mole-to-mole comparison to find the moles of the unknown substance. Finally, convert the moles of the unknown to the desired unit (grams, molecules, etc.). This process ensures accurate calculations involving enthalpy changes in chemical reactions.
 Created using AI
Created using AIWhat is the difference between a thermochemical equation and a regular stoichiometric equation?
The main difference between a thermochemical equation and a regular stoichiometric equation is the inclusion of the enthalpy change (ΔH) in thermochemical equations. While regular stoichiometric equations focus on the mole-to-mole relationships between reactants and products, thermochemical equations emphasize the relationship between ΔH and the moles of substances involved. This allows for the calculation of energy changes during chemical reactions, which is crucial for understanding thermochemistry.
 Created using AI
Created using AIHow do you calculate the enthalpy change (ΔH) for a reaction using a thermochemical equation?
To calculate the enthalpy change (ΔH) for a reaction using a thermochemical equation, follow these steps: 1) Write the balanced chemical equation with the ΔH value. 2) Identify the moles of reactants and products involved. 3) Use the stoichiometric coefficients to relate the moles of substances to the given ΔH. For example, if the equation is CH4 + 2O2 → CO2 + 2H2O, ΔH = -890 kJ, and you have 2 moles of CH4, the total ΔH would be 2 × -890 kJ = -1780 kJ.
 Created using AI
Created using AIWhy is it important to include ΔH in a thermochemical equation?
Including ΔH in a thermochemical equation is important because it provides information about the energy changes during a chemical reaction. This helps in understanding whether the reaction is exothermic (releases heat) or endothermic (absorbs heat). Knowing the ΔH value is crucial for applications in energy management, industrial processes, and predicting reaction behavior. It also allows for accurate calculations involving the conversion of energy to moles, grams, or other units, which is essential for practical and theoretical chemistry.
 Created using AI
Created using AIYour Introduction to Chemistry tutor
- The following equation shows the conversion of aluminum oxide (from the ore bauxite) to aluminum: 2 Al2O3(s) ...
- The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol (31.0 kJ/mol). How many kilojo...
- Converting liquid water to solid ice releases 1.44 kcal/mol (6.02 kJ/mol). How many kilocalories are released...
- Glucose, also known as 'blood sugar' when measured in blood, has the formula C6H12O6. What is the minimum amo...
- For the production of ammonia from its elements, ∆H = -22 kcal/mol(-19 kJ/mol). How much energy (in kilocalor...
- For the evaporation of water, H2O(l) → H2O(g), AT 100°C, ∆H = +9.72 kcal/mol (+40.7 kJ/mol). How many kilojo...
- Methanol, CH3OH, is used as race car fuel. How many kilojoules are released by burning 50.0 g of methanol?
