Osmosis is the net movement of a solvent, usually water, across a semipermeable membrane. Now, this semipermeable membrane is just the material that's allowing solvents and other small molecules to pass across and we're going to say that these cell membranes that are around living cells are in fact semipermeable themselves. Now, these cell membranes, they prevent solutes from passing through and these solutes can be ions or large molecules. Now, if we take a look here at this illustration of a semipermeable membrane, here the membrane is asking if you have an appointment. This larger molecule in red is trying to get through and they can't get past the barrier. But the smaller ones down here do have an appointment, are allowed to pass through because they are the right size, and they can pass through the semipermeable membrane to the other side. And this is the way that semipermeable membranes work. Osmosis is the movement of water, net movement of water, but semipermeable membranes can halt and stop certain molecules from traversing from one side to the other side of a living cell.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Osmosis: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIOsmosis is the net movement of water across a semipermeable membrane from a low solute concentration to a high solute concentration, driven by osmotic pressure. Tonicity describes the relative solute concentrations: hypotonic solutions have lower solute concentrations, isotonic solutions are equal, and hypertonic solutions have higher concentrations. In a hypotonic environment, cells may swell and burst (hemolysis), while in hypertonic environments, they may shrivel (crenation). Understanding these concepts is crucial for applications in biological systems and intravenous solutions.
Osmosis is the net movement of solvent across a semipermeable membrane.
Understanding Osmosis
Osmosis Concept 1
Video transcript
Osmosis Concept 2
Video transcript
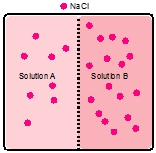
Now when it comes to osmosis, the solvent moves from a lower concentration solution to a higher concentration solution. Eventually, as it is doing this, equilibrium will be reached, and the net flow of the solvent is stopped by osmotic pressure. Osmotic pressure itself is the pressure exerted on the semipermeable membrane by the solvent. If we take a look here, we have these two images. Realize that the blue spheres are our solvent molecules, so think of them as water. And the red ones are our solute molecules. If we look at the image on the left, we can see that there are more red solute molecules on the top portion than on the bottom portion. That means the top part is more saturated, more concentrated. Because of this, remember, osmosis solvents want to move towards the higher concentration. That means that there's going to be greater osmotic pressure on this side here, which is going to force the water to go up towards the more concentrated side. So what's going to start happening is water is going to move through the semipermeable membrane and try to basically dilute this more concentrated portion. So we'd say that the flow of solvent is up.
Eventually, both sides are going to have the same type of concentration. It may not look like it, but just realize that the flow of water is going to dilute the top part, so it's going to be more volume per the same number of solute molecules. As a result of this, the pressure on both sides of the semipermeable membrane, this membrane, is going to be equal in force. So there's not going to be a net flow of water. So we're going to say here that the flow of solvent, the net flow would be 0. Right? We're going to say that there's not going to be a big change. Water is still flowing both ways, but one side is not gaining more water than the other side. So there's not a net change in volume for either side. So just remember, it's osmotic pressure that's going to stop this movement of water more so to one side than the other. This happens when the concentrations of both sides reach the same value. Initially, the more concentrated side is going to be diluted by the less concentrated side.
Osmosis Example 1
Video transcript
Here, osmosis is best defined as the movement of a solvent across a semipermeable membrane. Our solvent typically is water, so it's the movement of water molecules. So that means options b and c are out. And let's see. Across the semipermeable membrane into a region of low solute concentration? No. Not low. High. Because of that, option a can't be the answer. Therefore, option d is the correct one where water molecules move across the semipermeable membrane into a region of high solute concentration. So here, option d would be the correct answer.
A semipermeable membrane is placed between the following solutions.
Which solution will increase in volume?
Four U tubes each have distilled water in the right arm, a solution in the left arm, and a semipermeable membrane between the arms. If the solute is LiF, which solution is most concentrated?
Identify the direction of water flow between 2 solutions separates by semipermeable membrane, where 
Osmosis Concept 3
Video transcript
We know that osmosis is the movement of a solvent from an area of low concentration to an area of high concentration. Now, the direction of solvent flow depends on tonicity. Tonicity itself is just the relative concentration of solutes dissolved in the solutions. Now, when we take a look at the different types of solutions realize that we're looking at solute concentration and osmotic pressure relative to one another. If we take a look here we have our hypotonic solution, our isotonic solution, and our hypertonic solution. With a hypotonic solution, it has a lower solute concentration and lower osmotic pressure relative to bodily fluids. And when we talk about isotonic solutions, this is when two solutions have the same solute concentration and osmotic pressure. Now, an interesting piece of information is when we deal with intravenous solutions, they must be isotonic to bodily fluids such as blood, plasma, tissue cells, tissue fluids, etcetera. And then finally, we have hypertonic solutions which have a higher solute concentration and osmotic pressure relative to body fluids.
Now, if we take a look here, we're going to talk about hypotonic, isotonic and hypertonic environments and look at them in reference to their solute concentrations outside the cell, osmotic pressure outside the cell, and the effects they have on red blood cells. So when we're looking at hypotonic solutions, we're going to say they have lower solute concentration. We said isotonic they would have equal solute concentrations between two things being compared to one another. Hypertonic would have higher solute concentration. Now, what about their osmotic pressure outside the cell? Well, lower solute concentration would result in lower osmotic pressure. Here, the equal amount of solute concentration would equate to an equal osmotic pressure. Higher solute concentration will result in higher osmotic pressure. What effect does this have on a biological system such as a red blood cell? Well, here we're going to say that we have a lower concentration on the outside, so that would mean that it's more concentrated within the red blood cell. So remember, osmosis moves to where it's higher in concentration. So water would enter the cell. This causes a process known as hemolysis. So basically the cell will swell up because all the water is going in there, and if too much water gets in there it can burst. Okay, so a cell will swell and then could burst.
Next, we have an isotonic environment. So the concentration inside and outside the red blood cell are the same. So water enters and exits the cell at equal rates so there is no net movement of water. The same amount that goes in is the same amount that comes out. Finally, if we're in a hypertonic environment that means that it's more concentrated on the outside of the red blood cell, so water is going to exit the cell. If enough water exits the cell this causes crenation, so the cell will dehydrate and it shrivels.
Now, how's the way for us to remember these different types of situations? Well, here let's say we're looking at a hypotonic environment. So a hypotonic environment, hypo sounds close to hippo. Hippos drink, a hippo drinks too much water and swells like a cell. So hippos will take in a huge amount of water because the environment is hypotonic and they could burst or swell. A hypertonic environment, so the outside is more concentrated than the red blood cell. So a hypertonic environment can be related to a hyperkid. The hyperkid playing outside gets dehydrated like a cell. So if your environment is hypertonic, you're going to lose water out of your red blood cell. It's going to exit the red blood cell and try to dilute the outside environment. So just keep these little memory tools to help you know the distinction between hypotonic versus hypertonic. Isotonic, we know everything is equal on the inside and out so there's no net movement of water.

Osmosis Example 2
Video transcript
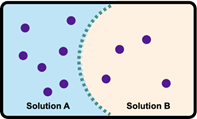
Here it says label the tonicity of the solution outside the cell where the dot, the purple dot, are these solute particles. Right. So what we have to do is we have to look at how many dots we have on the outside, and compare it to the number of dots on the inside. In the first image, we can see that inside the cell is more concentrated. Outside the cell is less concentrated, it has fewer purple dots. Remember, we have to discuss what type of solution is on the outside. Since the outside is less concentrated, it is a hypotonic solution. For the second one, both the inside and the outside have 5 purple dots, so 5 solute molecules. They're equal inside and out. So this would have to be an isotonic solution. And then finally here we have the outside having more dots. So outside is more concentrated. Since the outside is more concentrated, this represents a hypertonic solution. So just remember, we're looking at what the solution is on the outside relative to what's inside a particular cell.
If the fluid surrounding a patient's red blood cells is depleted in electrolytes, is crenation or hemolysis more likely to occur?
A solution with the same osmotic pressure as the blood is
A red blood cell placed in pure water will swell because:
Here’s what students ask on this topic:
What is osmosis and how does it work?
Osmosis is the net movement of water across a semipermeable membrane from an area of low solute concentration to an area of high solute concentration. This process is driven by osmotic pressure, which is the pressure exerted by the solvent on the semipermeable membrane. The membrane allows only certain small molecules, like water, to pass through while blocking larger molecules and ions. As water moves to balance the solute concentrations on both sides of the membrane, it continues until equilibrium is reached, where the osmotic pressure on both sides is equal, resulting in no net flow of water.
 Created using AI
Created using AIWhat is the difference between hypotonic, isotonic, and hypertonic solutions?
Hypotonic, isotonic, and hypertonic solutions describe the relative solute concentrations compared to another solution, often bodily fluids. A hypotonic solution has a lower solute concentration and lower osmotic pressure, causing cells to swell and potentially burst (hemolysis) as water enters. An isotonic solution has equal solute concentration and osmotic pressure, resulting in no net movement of water into or out of cells. A hypertonic solution has a higher solute concentration and higher osmotic pressure, causing cells to lose water and shrivel (crenation) as water exits to dilute the external environment.
 Created using AI
Created using AIHow does osmotic pressure affect the movement of water in osmosis?
Osmotic pressure is the force exerted by the solvent on a semipermeable membrane due to solute concentration differences. In osmosis, water moves from an area of low solute concentration to high solute concentration to balance the solute levels. As water moves, it increases the osmotic pressure on the side with higher solute concentration. This pressure continues to build until equilibrium is reached, where the osmotic pressure on both sides of the membrane is equal, stopping the net flow of water. Thus, osmotic pressure is crucial in driving and eventually halting the movement of water in osmosis.
 Created using AI
Created using AIWhat happens to red blood cells in hypotonic and hypertonic environments?
In a hypotonic environment, the solute concentration outside the red blood cells is lower than inside, causing water to enter the cells. This influx of water can lead to the cells swelling and potentially bursting, a process known as hemolysis. In a hypertonic environment, the solute concentration outside the red blood cells is higher than inside, causing water to exit the cells. This loss of water results in the cells shrinking and shriveling, a process called crenation. Both conditions can be detrimental to cell function and survival.
 Created using AI
Created using AIWhy must intravenous solutions be isotonic to bodily fluids?
Intravenous (IV) solutions must be isotonic to bodily fluids to prevent adverse effects on cells. If an IV solution is hypotonic, it can cause cells to swell and burst (hemolysis) as water enters the cells. Conversely, if the IV solution is hypertonic, it can cause cells to lose water and shrivel (crenation). An isotonic IV solution has the same solute concentration and osmotic pressure as bodily fluids, ensuring that there is no net movement of water into or out of cells, maintaining cellular integrity and function.
 Created using AI
Created using AIYour Introduction to Chemistry tutor
- Assume that two liquids are separated by a semipermeable membrane, with pure solvent on the right side and a s...
- What does it mean when we say that a 0.15 M NaCl solution is isotonic with blood, whereas distilled water is h...
- Look up the composition of Ringer's solution used in the treatment of burns and wounds. What is the osmolarit...
- Research information related to dialysis and answer the following questions: What is the difference between h...