Now, Lewis dot structures are structural representations of elements that use valence electrons to form their covalent bonds. Now, we're going to say that there are many possible Lewis bond structures that exist, but there are rules to draw the best structure. We're going to say, recall elements form bonds in order to gain electrons and become like the nearest noble gas. So when we're drawing these Lewis dot structures, we're going to go through a series of rules that help us to illustrate the best connections for those particular Lewis dot structures, also known as molecular compounds.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Lewis Dot Structures: Neutral Compounds (Simplified): Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AILewis dot structures represent elements using their valence electrons to illustrate covalent bonds. These structures help visualize how atoms bond to achieve a stable electron configuration, similar to noble gases. To draw the most effective Lewis structures, one must follow specific rules that guide the arrangement of electrons and bonds in molecular compounds. Understanding these principles is essential for grasping concepts like molecular geometry and the behavior of different chemical species in reactions.
Lewis Dot Structures or Electron Dot Structures are diagrams that show how elements in a molecule use their valence electrons to form bonds.
Lewis Dot Structures
Lewis Dot Structures: Neutral Compounds (Simplified) Concept 1
Video transcript
Lewis Dot Structures: Neutral Compounds (Simplified) Example 1
Video transcript
Here it says we need to draw the Lewis dot structure for the silicon tetrabromide molecule, which is SiBr4. To do that, we're going to take a look at the following rules. Alright. So step 1 says that we need to determine the total number of valence electrons of the structure. Now recall, the total number of valence electrons equals the group number of the element. So here we have silicon, which is in group 4A, and there's one of it. And then here we're going to say we have bromines. Bromines are in group 7A, so there are 7 valence electrons each and there are 4 of them. So 28 + 4 gives me 32 total valence electrons within this structure. Alright. Step 2, we're going to place the least electronegative element in the center and connect all elements with single bonds. Alright. So we're going to say silicon is less electronegative than bromine. So we're going to connect silicon to the 4 bromines. Now remember, silicon's in group 4 so it contributes 4 valence electrons here. And remember each single bond has in it 2 valence electrons. So here goes the other electron on the other end. Alright. To do this, remember, we're going to follow the bonding preferences guide to determine atom connectivity. We know this makes sense because silicon is in group 4A, elements in group 4A want to make 4 bonds. Step 3, we're going to add electrons to all surrounding elements until they have 8 electrons, which we refer to as the octet rule. But remember, we also have the duet rule when it comes to hydrogen. Hydrogen only wants 2 valence electrons around it because doing so gets us the same configuration as helium. Right. Now, we're going to add all the electrons that we can. So we've already used 8 electrons. Right? So that means that we have what? 24 electrons remaining. So 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24. So we've used all 24 remaining electrons, so we have 0 left. So step 4 you don't have to do. Step 4 says we place any remaining electrons on the central atom. In this case, we don't have any electrons remaining and this would be our structure. We'd have silicon making 4 bonds, it would have 0 lone pairs on it, each bromine is making a single bond, and each one has 3 lone pairs on it. So this would be the structure for our silicon tetrabromide molecule.
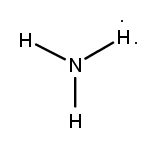
Determine the Lewis Dot Structure for the NH3 compound.
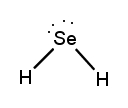
Determine the Lewis Dot Structure for the following compound:H2Se.
Draw a Lewis Dot Structure that obeys the octet rule for the following compound:NH2OH.
Here’s what students ask on this topic:
What are Lewis dot structures and why are they important in chemistry?
Lewis dot structures are diagrams that represent the valence electrons of atoms within a molecule. These structures are crucial because they help visualize how atoms bond covalently to achieve a stable electron configuration, similar to noble gases. By showing the arrangement of electrons, Lewis dot structures allow chemists to predict the molecular geometry, reactivity, and properties of compounds. Understanding these structures is essential for grasping more complex concepts in chemistry, such as molecular geometry, polarity, and the behavior of different chemical species in reactions.
 Created using AI
Created using AIWhat are the rules for drawing Lewis dot structures?
To draw Lewis dot structures, follow these steps: 1) Count the total number of valence electrons for all atoms in the molecule. 2) Write the symbols for the atoms, arranging them to show which atoms are connected. 3) Use a pair of electrons to form a bond between each pair of bonded atoms. 4) Distribute the remaining electrons to satisfy the octet rule (or duet rule for hydrogen) for each atom. 5) If there are not enough electrons to satisfy the octet rule, form double or triple bonds as necessary. These rules help ensure the most stable and accurate representation of the molecule.
 Created using AI
Created using AIHow do you determine the central atom in a Lewis dot structure?
The central atom in a Lewis dot structure is typically the least electronegative element, excluding hydrogen, which is always a terminal atom. To determine the central atom, compare the electronegativities of the atoms involved; the one with the lowest electronegativity is usually placed in the center. This atom is then bonded to the surrounding atoms. For example, in CO2, carbon is the central atom because it is less electronegative than oxygen.
 Created using AI
Created using AIWhat is the octet rule and how does it apply to Lewis dot structures?
The octet rule states that atoms tend to form bonds until they are surrounded by eight valence electrons, achieving a stable electron configuration similar to noble gases. In Lewis dot structures, this rule guides the placement of electrons around atoms. For example, in a water molecule (H2O), oxygen shares electrons with two hydrogen atoms to complete its octet. However, there are exceptions, such as molecules with an odd number of electrons, molecules where one or more atoms possess more or fewer than eight electrons, and molecules with atoms that have d-orbitals available for bonding.
 Created using AI
Created using AIHow do you represent double and triple bonds in Lewis dot structures?
In Lewis dot structures, double and triple bonds are represented by two or three pairs of shared electrons between two atoms, respectively. For example, in the molecule O2, a double bond is shown by two pairs of dots or a double line between the oxygen atoms. In N2, a triple bond is represented by three pairs of dots or a triple line between the nitrogen atoms. These multiple bonds are used when single bonds do not satisfy the octet rule for the atoms involved.
 Created using AI
Created using AIYour Introduction to Chemistry tutor
- CIA Problem 4.2 Draw the Lewis dot structures for the molecules CO and NO. What is different about these struc...
- How many covalent bonds are formed by each atom in the following molecules? Draw molecules using the electron-...
- Write electron-dot symbols to show the number of covalent bonds and the lone pairs of electrons in the molecul...
- Distinguish between the following: c. A lone pair and a shared pair of elecctrons
- Consider the following possible structural formulas for C₃H₆O₂ . If a structure is not reasonable, explain wha...
- Expand the following condensed structures into the correct structural formulas. c. CH₃CH₂OCH₂Cl
- The following formulas are unlikely to be correct. What is wrong with each? d. C₂OS
- The phosphonium ion, PH⁺₄ is formed by reaction of phosphine, PH₃ , with an acid. d. Explain why the ion has a...
- State the number of valence electrons, bonding pairs, and lone pairs in each of the following Lewis structures...
- Draw the Lewis structure for each of the following: (6.6) b. H₂NOH N is the central atom)
- Draw the Lewis structure for each of the following: (6.6) a. H₃COCH₃ (the atoms are in the order C O C)