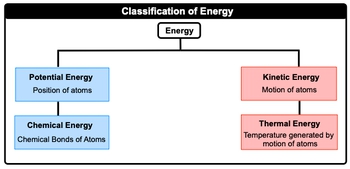
Thermal chemistry is the study of matter and energy associated with chemical reactions or physical changes. Energy itself is just the capacity to do work or to produce heat. Now when we talk about energy, realize that there are different types of energy, but in this chapter, we're only going to focus on a selected few. Now when we take a look at the big picture, energy itself, we can say here that it can be broken down initially into either the position of atoms or the motion of atoms. When we're talking about the position of atoms, this is just simply our potential energy, our energy of position. And then if we're talking about the movement or motion of atoms, this is connected to kinetic energy. Now both potential energy and kinetic energy can be further broken down into other types of energy. Potential energy, we can connect it to another one which deals with the chemical bonds of atoms. This is just simply chemical energy. And then kinetic energy can be broken down further into energy associated, with temperature generated by the motion of atoms. So this would be called thermal energy. So just remember, energy is just the capacity to work and to produce heat. And when we're talking about energy in this chapter, we're mainly concerned with these different types of energy forms. Now, of course, there are other types of energy that exist. We'll go into those in later chapters, but for now, just remember these particular four.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Nature of Energy: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIThermal chemistry examines the energy changes during chemical reactions and physical changes. Energy, defined as the capacity to do work or produce heat, can be categorized into potential energy, related to atomic position, and kinetic energy, associated with atomic motion. Key forms include chemical energy and thermal energy. The SI unit for energy is the Joule, with conversion factors such as 1 calorie = 4.184 joules and 1 kilowatt-hour = 3.6 × 106 joules, essential for calculations in thermochemistry.
Thermochemistry is the study of matter and energy associated with chemical reactions or physical changes.
Classification of Energy
Nature of Energy
Video transcript
Nature of Energy
Video transcript
In our discussion of the concept of energy, it's important to remember that the SI unit for energy is Joule, and it's named after the English scientist James Joule. When it comes to joules, we're going to say that there are 3 particular types of conversion factors associated with it.
We're going to say here for the first one, we have one calorie which is lowercase c; this equals 4.184 joules. Next, we have 1 capital C calorie, this one is associated with food nutrition. This particular calorie equals 4,184 joules. And then finally, kilowatt hours. Usually, when we talk about an electrical bill, it's associated with kilowatt hours. So we're gonna say here kilowatt hours equal 3.6×106 joules.
So these are 3 conversion factors associated with our SI unit for energy. Also, realize here that I didn't put purple boxes around them, so usually, you're not expected to memorize them. They're often given to you within the question or they're given to you on a formula sheet when taking an exam. But here, just again remember, these are 3 common types of conversion factors associated with Joule's.

Nature of Energy Example 1
Video transcript
Here it states, which of the following statements deals with potential energy with no chemical energy associated with it. Remember, potential energy is just the energy of position, whereas chemical energy, an offshoot of potential energy, is associated with the chemical bonds of atoms. Alright. So if we take a look here, it says a car traveling with a velocity of 51 meters per second with a mass of 1,250 kilograms. Here we're talking about velocity, we're talking about the motion of this car, so this is more associated with kinetic energy. So this is out. Next, your chemistry book sitting on a table counter near the trash can weighing 12 newtons at a height of 1.2 meters. So make sure it doesn't fall into the trash can. Here they're just talking about the position of the chemistry book. It's at a certain height, and then we're talking about a force on it. Here, this is purely potential energy, so this would be our answer. But let's look at the other options. A chunk of coal being thrown into a furnace to generate heat. Here we're talking about heat being generated from this. This is closely related to thermal energy. And then next, we have the warmth coming from a campfire. So again, we're talking about temperature, we're talking about heat. This is a version of kinetic energy in the form of thermal energy. So out of all the choices, the only one that is purely potential energy without talking about chemical energy would have to be option B.
An energy efficient refrigerator uses 780 kWh of electrical energy per year. How many kilocalories of electricity does it use in three years?
2.0 x 105 kcal
8.3 x 105 kcal
2.0 x 106 kcal
8.3 x 106 kcal
Here’s what students ask on this topic:
What is the definition of energy in thermochemistry?
In thermochemistry, energy is defined as the capacity to do work or to produce heat. It is a fundamental concept that helps us understand the changes that occur during chemical reactions and physical changes. Energy can be categorized into two main types: potential energy, which is related to the position of atoms, and kinetic energy, which is associated with the motion of atoms. These categories can be further broken down into specific forms such as chemical energy (a type of potential energy) and thermal energy (a type of kinetic energy).
 Created using AI
Created using AIWhat are the different types of energy discussed in thermochemistry?
In thermochemistry, the primary types of energy discussed are potential energy and kinetic energy. Potential energy is related to the position of atoms and includes chemical energy, which is the energy stored in chemical bonds. Kinetic energy is associated with the motion of atoms and includes thermal energy, which is the energy generated by the motion of atoms and is related to temperature. These forms of energy help us understand how energy is transferred and transformed during chemical reactions and physical changes.
 Created using AI
Created using AIWhat is the SI unit for energy and its common conversion factors?
The SI unit for energy is the Joule (J), named after the English scientist James Joule. Common conversion factors for Joules include: 1 calorie (lowercase 'c') = 4.184 Joules, 1 Calorie (uppercase 'C', used in food nutrition) = 4,184 Joules, and 1 kilowatt-hour (kWh) = 3.6 × 106 Joules. These conversion factors are essential for calculations in thermochemistry and are often provided in exam questions or formula sheets.
 Created using AI
Created using AIHow is thermal energy related to kinetic energy?
Thermal energy is a form of kinetic energy. It is the energy associated with the motion of atoms and molecules within a substance. As the motion of these particles increases, the thermal energy of the substance also increases, which is often observed as an increase in temperature. In thermochemistry, understanding thermal energy is crucial for analyzing how energy is transferred and transformed during chemical reactions and physical changes.
 Created using AI
Created using AIWhat is the difference between potential energy and kinetic energy in the context of thermochemistry?
In thermochemistry, potential energy is the energy related to the position of atoms or molecules. It includes chemical energy, which is stored in the bonds between atoms. Kinetic energy, on the other hand, is the energy associated with the motion of atoms or molecules. Thermal energy, a type of kinetic energy, is related to the temperature of a substance. The key difference is that potential energy is about position and stored energy, while kinetic energy is about motion and active energy.
 Created using AI
Created using AIYour Introduction to Chemistry tutor
- Discuss the changes in the potential and kinetic energy of a roller-coaster ride as the roller-coaster car cli...
- Indicate whether each of the following statements describes potential or kinetic energy: b. kicking a ball
- Using the energy values for foods (see TABLE 3.7), determine each of the following (round off the answer for e...
- Using the energy values for foods (see TABLE 3.7), determine each of the following (round off the answer for e...
- For dinner, Charles had one cup of clam chowder, which contains 16 g of carbohydrate, 12 g of fat, and 9 g of ...
- A patient receives 3.2 L of intravenous (IV) glucose solution. If 100. mL of the solution contains 5.0 g of gl...
- What is a catalyst, and what effect does it have on the activation energy of a reaction?
- If a catalyst changes the activation energy of a forward reaction from 28.0 kcal/mol to 23.0 kcal/mol, what ef...
- Using energy values from TABLE 3.8, determine each of the following: c. If Charles consumes 1800 kcal per day...
- b. For the amount of exercise that Charles did for one week in part a, if expending 3500 kcal is equal to a lo...
- For the unbalanced combustion reaction shown, 1 mol of ethanol, C2H5OH, releases 327 kcal (1370 kJ): C2H5OH ...
- A 70.0-kg person had a quarter-pound cheeseburger, french fries, and a chocolate shake. (3.5) d. Using TABL...
- The relationship between the nutritional unit for energy and the metric unit is 1 calorie = 1 kcal. a. One don...
- When a 0.66-g sample of olive oil is burned in a calorimeter, the heat released increases the temperature of 3...
- A patient is receiving 3000 mL/day of a solution that contains 5 g of dextrose (glucose) per 100 mL of solutio...
