Textbook Question
Classify the following carbohydrates as a monosaccharide, disaccharide, oligosaccharide, or polysaccharide:
(a) raffinose, a soluble fiber containing three carbohydrate units
29
views
 Verified step by step guidance
Verified step by step guidance
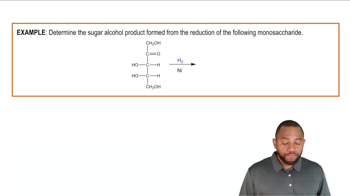


Classify the following carbohydrates as a monosaccharide, disaccharide, oligosaccharide, or polysaccharide:
(a) raffinose, a soluble fiber containing three carbohydrate units
Identify the following as characteristics of soluble or insoluble fiber:
(a) can mix with water
Identify the following as containing soluble or insoluble fiber:
(a) oatmeal
Use the structure of d-galactose in Problem 6.15 to answer the following:
(b) Draw the Fischer projection of l-galactose.
ALLIED Health Indicate whether the following statements apply to type 1 or type 2 diabetes:
(c) can be managed with diet and exercise
ALLIED Health Indicate whether the following statements apply to type 1 or type 2 diabetes:
(a) most cases begin in youth