A dioxin-contaminated water source contains 0.085% dioxin by mass. How much dioxin is present in 2.5 L of this water? Assume a density of 1.00 g/mL.
Describe how to prepare each solution from the dry solute and the solvent. c. 125 g of 1.0% NaNO3 solution by mass
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Mass Percent Concentration

Solution Preparation

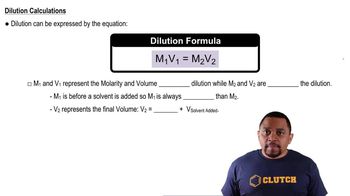
Dilution and Mixing
Lead is a toxic metal that affects the central nervous system. A Pb-contaminated water sample contains 0.0011% Pb by mass. How much of the water (in mL) contains 150 mg of Pb? (Assume a density of 1.0 g/mL.)
Describe how to prepare each solution from the dry solute and the solvent. b. 125 g of 0.100 m NaNO3
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. a. molarity
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. b. molality
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. c. percent by mass
