Describe how to prepare each solution from the dry solute and the solvent. b. 125 g of 0.100 m NaNO3
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. b. molality
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Concentration Units
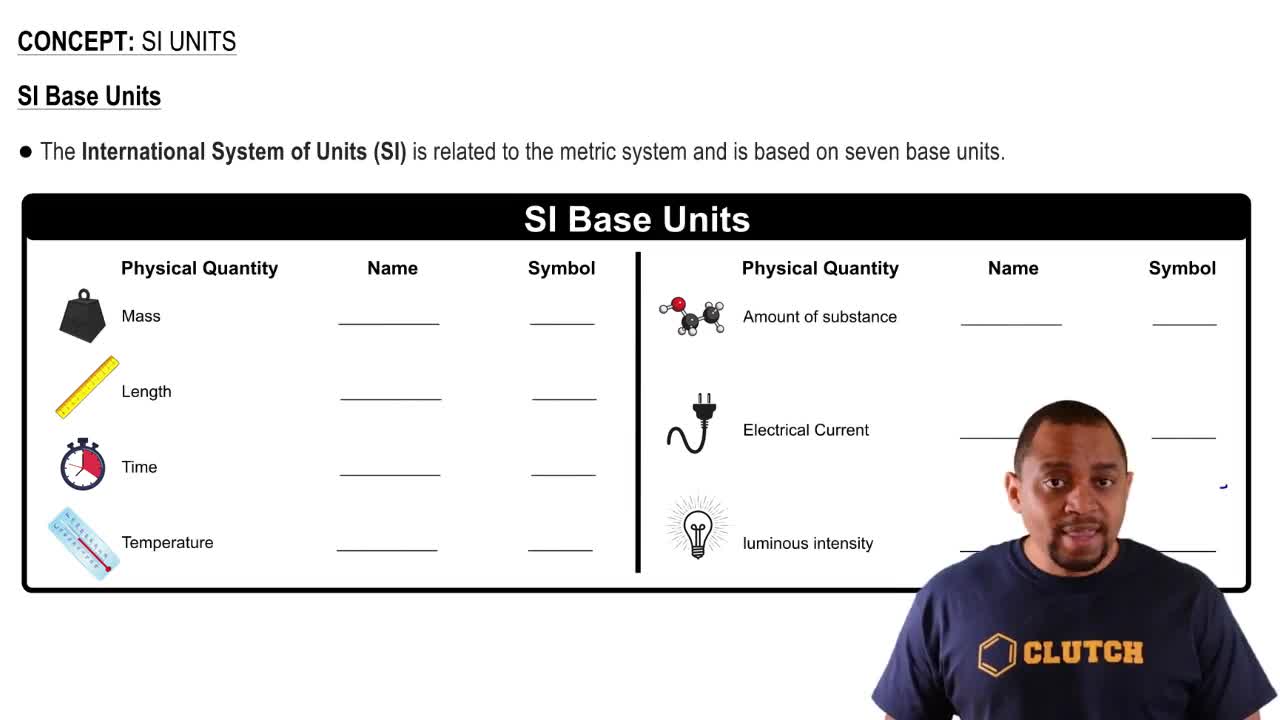
Molarity vs. Molality

Density and Volume Calculations

Describe how to prepare each solution from the dry solute and the solvent. c. 125 g of 1.0% NaNO3 solution by mass
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. a. molarity
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. c. percent by mass
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. d. mole fraction
A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 °C. The final volume of the solution is 118 mL. The densities of methanol and water at this temperature are 0.782 g/mL and 1.00 g/mL, respectively. For this solution, calculate the concentration in each unit. e. mole percent
