A gas has a Henry's law constant of 0.112 M>atm. What total volume of solution is needed to completely dissolve 1.65 L of the gas at a pressure of 725 torr and a temperature of 25 °C?
An isotonic solution contains 0.90% NaCl mass to volume. Calculate the percent mass to volume for isotonic solutions containing each solute at 25 °C. Assume a van't Hoff factor of 1.9 for all ionic solutes. a. KCl

Verified Solution
Key Concepts
Isotonic Solutions

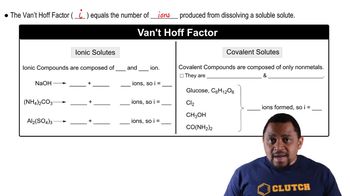
Van't Hoff Factor (i)
Percent Mass to Volume Concentration

The Safe Drinking Water Act (SDWA) sets a limit for mercury—a toxin to the central nervous system—at 0.0020 ppm by mass. Water suppliers must periodically test their water to ensure that mercury levels do not exceed this limit. Suppose water becomes contaminated with mercury at twice the legal limit (0.0040 ppm). How much of this water would a person have to consume to ingest 50.0 mg of mercury?
Water softeners often replace calcium ions in hard water with sodium ions. Since sodium compounds are soluble, the presence of sodium ions in water does not cause the white, scaly residues caused by calcium ions. However, calcium is more beneficial to human health than sodium because calcium is a necessary part of the human diet, while high levels of sodium intake are linked to increases in blood pressure. The U.S. Food and Drug Administration (FDA) recommends that adults ingest less than 2.4 g of sodium per day. How many liters of softened water, containing a sodium concentration of 0.050% sodium by mass, would a person have to consume to exceed the FDA recommendation? (Assume a water density of 1.0 g/mL.)
Magnesium citrate, Mg3(C6H5O7)2, belongs to a class of laxatives called hyperosmotics, which cause rapid emptying of the bowel. When a concentrated solution of magnesium citrate is consumed, it passes through the intestines, drawing water and promoting diarrhea, usually within 6 hours. Calculate the osmotic pressure of a magnesium citrate laxative solution containing 28.5 g of magnesium citrate in 235 mL of solution at 37 °C (approximate body temperature). Assume complete dissociation of the ionic compound.
When HNO2 is dissolved in water, it partially dissociates according to the equation HNO2 ⇌ H+ + NO2-. A solution is prepared that contains 7.050 g of HNO2 in 1.000 kg of water. Its freezing point is -0.2929 °C. Calculate the fraction of HNO2 that has dissociated.
A solution of a nonvolatile solute in water has a boiling point of 375.3 K. Calculate the vapor pressure of water above this solution at 338 K. The vapor pressure of pure water at this temperature is 0.2467 atm.
