Here are the essential concepts you must grasp in order to answer the question correctly.
Structural Isomers
Structural isomers are compounds that have the same molecular formula but differ in the arrangement of atoms. This variation can lead to different physical and chemical properties. In the case of heptane (C7H16), the nine structural isomers arise from different ways to connect the carbon atoms, resulting in distinct structures.
Recommended video:
Heptane and its Isomers
Heptane is an alkane with seven carbon atoms, and its molecular formula is C7H16. The structural isomers of heptane include straight-chain and branched forms. Understanding the specific arrangements of carbon and hydrogen in these isomers is crucial for drawing their structural formulas accurately.
Recommended video:
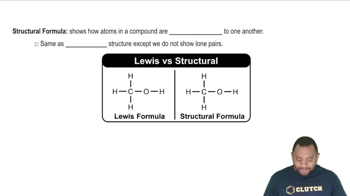
Drawing Structural Formulas
Drawing structural formulas involves representing the arrangement of atoms in a molecule, including bonds between them. For isomers, it is important to depict the connectivity of carbon atoms and the placement of hydrogen atoms. Familiarity with line-angle formulas and the conventions for depicting bonds helps in accurately illustrating the different structural isomers of heptane.
Recommended video: