Here are the essential concepts you must grasp in order to answer the question correctly.
Alcohol Functional Group
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups attached to a carbon atom. The hydroxyl group is responsible for the chemical properties of alcohols, including their ability to form hydrogen bonds, which affects their boiling points and solubility in water.
Recommended video:
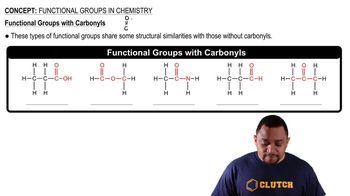
Carbonyl Functional Groups
Nomenclature of Alcohols
The naming of alcohols follows the IUPAC system, where the longest carbon chain containing the hydroxyl group is identified, and the suffix '-ol' is added to the name. The position of the hydroxyl group is indicated by a number, which corresponds to the carbon atom to which it is attached, ensuring clarity in the compound's structure.
Recommended video:
Rules for Naming Alcohols
Structural Representation of Organic Compounds
The structural formula of organic compounds visually represents the arrangement of atoms within the molecule. In the provided image, the carbon chain is depicted with hydrogen atoms implied, and the hydroxyl group is shown at the end, indicating that this is a straight-chain alcohol. Understanding structural formulas is essential for identifying functional groups and predicting chemical behavior.
Recommended video:
Introduction to Organic Chemistry