Here are the essential concepts you must grasp in order to answer the question correctly.
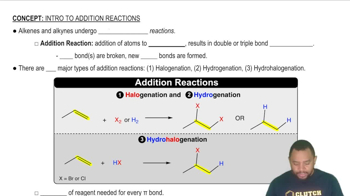
Addition Reactions
Addition reactions are a type of chemical reaction where two or more reactants combine to form a single product. This process typically involves unsaturated compounds, such as alkenes or alkynes, which have double or triple bonds that can be broken to allow new bonds to form. Understanding the mechanism of these reactions is crucial for predicting the products formed.
Recommended video:
Functional Groups
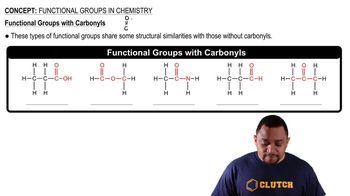
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In addition reactions, the presence of certain functional groups, such as alkenes, can significantly influence the outcome of the reaction. Identifying these groups helps in determining the expected products of the reaction.
Recommended video:
Carbonyl Functional Groups
Reaction Mechanisms
Reaction mechanisms describe the step-by-step sequence of elementary reactions by which overall chemical change occurs. In the context of addition reactions, understanding the mechanism allows chemists to predict the formation of products, including stereochemistry and regioselectivity. This knowledge is essential for accurately determining the final product of the reaction.
Recommended video:
Reaction Mechanism Overview