Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization of Acids
Ionization refers to the process by which an acid donates protons (H+) to water, forming hydronium ions (H3O+) and its conjugate base. For weak acids like benzoic acid, this process is not complete, and only a fraction of the acid molecules ionize in solution. Understanding this concept is crucial for calculating the percent ionization.
Recommended video:
Calculating Percent Ionization of Weak Acids
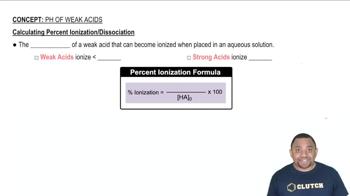
Percent Ionization
Percent ionization is a measure of the extent to which an acid ionizes in solution, expressed as a percentage. It is calculated using the formula: (concentration of ionized acid / initial concentration of acid) × 100%. This concept helps quantify the strength of an acid in solution and is particularly important for weak acids.
Recommended video:
Percent Ionization Example
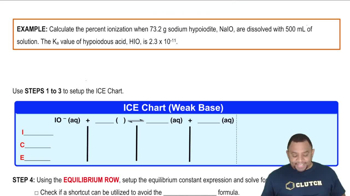
Equilibrium Constant (Ka)
The acid dissociation constant (Ka) quantifies the strength of an acid in solution by measuring the equilibrium concentrations of the products and reactants in the ionization reaction. For benzoic acid, knowing the Ka value allows for the calculation of the concentration of ionized species at equilibrium, which is essential for determining percent ionization.
Recommended video: